Min_HW02_Oxy_Anions
advertisement

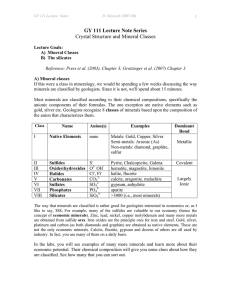
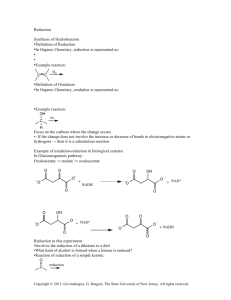
Homework #2 – Mineralogy Geos 250 Name:________________________ Bridging Oxygens – Linking Molecular Oxy-anion Groups: Borates & Silicates 1 Other poly-“period 2”-ates, eg, carbonate, nitrate, borate: These chemical names and types are not inorganic mineral solids like the linked borate triangles in borate salts or linked silicate tetrahedra in silicate minerals. Instead they use a single (NO3)- or (BO3)- or (CO3)-2 ion and substitute an oxygens for a carbon or hydrogen to link 2 more complex organic molecule (with chains, rings, methyl side groups, and hetero elements (N, O, S) to make a polymer. These dimers joined by a carbonate group etc. then stick to each other the way that organic molecules do by polarizing each other and condensing to a higher molecular weight solid (the way C22H46 paraffin condenses to make wax candles instead of individual oily strings). Don’t infer that a polycarbonate pop bottle or fleece jacket is made of strings of limestone! Because only borates and silicates do the bridging oxy-anion thing, only they get to be structure formers in natural minerals. N-O compounds polymerize as gases such as NO2 N2O4 , nitrogen dioxide polymerizes to form di-nitrogen tetra-oxide. In thins case unpaired electrons and polarization are involved rather than bridging oxygens shared between adjacent groups so it isn’t the same thing as in mineral polymeric oxyanions. The borates triangles and silicate tetrahedral are unique in this regard. Because of this bridging oxygen structure, borate and silicate melts are completely intersoluble and it is possible to use borate as a flux to make low melting point softer boro-silicate glasses. It is also common to find solid borosislicate minerals like the tourmaline family. Because of variations in bridging oxygens and in the size of cation interstices, the chemical formulae for these minerals are highly variable and they tolerate a lot of subsittutions. 2 3 4 5 6