Silicates and Silicones Chemistry: Structures & Applications
advertisement

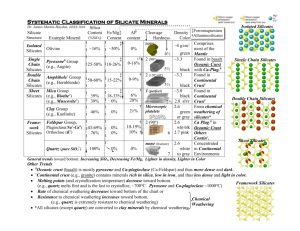
CHEMISTRY OF SILICATES AND SILICONES Dr H Govender/ Dr B Moodley 2020 • Earth’s crust – composed of about 95 % of silicate minerals, aluminosilicate clays, or silica which makes up the bulk of rocks, sand, and their breakdown products sand and clay. • Building materials are mainly silicates: slate, granite, brick, cement, ceramics and glass. • O, Si, and Al the three most abundant elements that makes up the earth’s crust – together they make up 81 % of the earth’s crust. Soluble silicates Silicates preparation - fusing an alkali metal carbonate with sand in an electric furnace at approximately 1400 oC. Na2CO3 CO2 + Na2CO3 1400o C SiO 2 Na4SiO4, (Na2SiO3)n • Product – soluble glass of sodium or potassium silicate • Dissolved in hot water under pressure • Used in detergent preps to keep pH high, removing grease and fats forming soap. • Soluble silicates must not be used when the water is hard – will react with Ca2+ to form insoluble calcium silicates. • Sodium silicate used as an adhesive, in asbestos roof tiles, in fireproof paint and putty, and in making silica gel. Silicate Structures • Majority of silicate minerals are insoluble – due to an infinite ionic structure and the strong Si-O bond. • Difficult to study their structures. • Structures were solved by X-ray crystallography. • Electronegativity difference between O and Si, 3.5 – 1.8 = 1.7, suggests that the bonds are 50 % ionic and 50 % covalent. Classification of silicates • The way in which (SiO4)4- tetrahedral units are linked together provides a convenient classification of silicate minerals. • Orthosilicates (neso-silicates) • - A wide variety of minerals contain discrete SiO44- tetrahedral units. - They have the formula: M2II[SiO4] where M = Be, Mg, Fe, Mn, or Zn. - Zircon, ZrSiO4- used as a gemstone – cut to look like diamond but is much softer than diamond. • Pyrosilicate (soro-silicates, disilicates) - Simplest of the condensed silicate ions which are composed of two tetrahedral units joined by sharing the O at one corner, giving the unit (Si2O7)6- Pyrosilicates are rare, e.g. thortveitite Sc2[Si2O7] Si2O7 6- • Cyclic Silicates - Made up of ring structures of the formula (SiO3)n2n-. - Examples include: Si3O96- occurs in wollastonite Ca3[Si3O9] and in benitoite BaTi[Si3O9]. - Si6O18 occur in beryl (a pale green gemstone) Be3Al2[Si6O18] and emerald (same formula as beryl except that it contains 1-2% Cr) • Chain Silicates - Simple chain silicates or pyroxenes – formula (SiO3)n2n-. • Sheet silicates (phyllo-silicates) - SiO4 units share three corners forming an infinite 2-d sheet of empirical formula (Si2O5)n2n-. - There are strong bonds within the Si-O sheet, but much weaker forces hold each sheet next to each other. - Other well known sheet silicates: (i). (ii). (ii). (iv). Clay minerals (kaolinite, pyrophylllite, talc) White asbestos (chrysotile, biotite) Micas (muscovite and margarite) Montmorillonites (Fullers earth, bentonite and vermiculite) • 3-d Silicates - Examples: SiO2 (quartz, tridymite, cristobalite etc). - Generally no metal ions, but 3-d structures can form the basis of silicate structures if there is replacement of some of the Si4+ by Al3+. - Zeolites are examples of 3-d silicates e.g. Na2[Al2Si3O10]2H2O – used as ion exchange material (https://www.youtube.com/watch?v=w7tUZIVz5LE) and as molecular sieves (A molecular sieve is a material with pores of uniform size. These pore diameters are similar in size to small molecules, and thus large molecules cannot enter or be adsorbed, while smaller molecules can. (Link zeolites : https://youtu.be/g351-MEeAJU-) play video here **** Molecular sieves 4 Angstrom Silicates in Technology • Alkali silicates (see soluble silicates) • Cement - Silicate are important components of both Portland cement and high alumina cement (. This type of cement is formed by calcining bauxite and lime. The bauxite is an aluminium ore). • Ceramics - Ceramics are inorganic materials that can made into a paste and shaped at normal temp: the shape is then fired at high temp - A number of carbides, oxides and in particular clays are treated in this way. - This process is used to make bricks, tiles, and pottery. - On heating kaolinite Al2(OH)4[Si2O5] loses water at 500-600 oC giving Al2O3∙2SiO2 and at about 950 oC forms a solid solution mullite (3 Al2O3∙2SiO2) and SiO2. • Glass - A small amount of glass is made of silica, has excellent properties but very high temperatures are needed to make it. - Silica glass is too expensive for general use. - The following oxides may be used in making silica glass, Na2O, K2O, MgO, CaO, BaO, B2O3, Al2O3, PbO and ZnO. - If only Na2O and K2O were used the glass would be water soluble. - Normal domestic glass for windows is a calcium alkali silicate glass made by fusing the alkali metal carbonate, CaCO3 and SiO2. - If Na2CO3 were used we obtain soda glass, and K2CO3 produces potash glass. - Most of the CaO maybe replaced by PbO giving lead glass, which has a higher refractive index and is used for making optical parts and glass ornaments. - If Al2O3 is used, Al3+ maybe present in the structure as a free metal ion, or it may replace Si4+ in SiO4 tetrahedra. - If B2O3 is used, B3+ replaces some Si4+ → borosilicate glass. - They have low coeffiecient of expansion and can withstand heat changes without cracking. - Such glassware contain less alkali and are less prone to chemical attack and are used as laboratory equipment such as Pyrex glassware. Organosilicon compounds and Silicones 1. Organosilicon compounds - Si-C bonds are as almost as strong as C-C bonds - SiC is extremely hard and stable. - Many organosilicon compounds have been made which are inert and stable to heat. Preparation of Organosilicon compounds • By Grignard Reaction SiCl4 + CH3MgCl →CH3SiCl3 + MgCl2 • Using an organolithium compound 4 LiR + SiCl4 → SiR4 + 4 LiCl (R = alkyl or aryl substituent) • Rochow ‘Direct Process Si + 2 CH3Cl Cu catalyst 280 – 300oC (CH3)2SiCl2 • Catalytic addition of Si-H to an alkene. - This is a useful general method, but is not applicable to making methyl and phenyl silanes required by the silicone industry. 2. Silicones • Are a group of organosilicon polymers. • Commercial uses include: fluids, oils, elastomers (rubbers) and resins. • Complete hydrolysis of SiCl4 yields SiO2, which has a very stable 3-d structure. • Research by F.S. Kipping on hydrolysis of alkyl substituted chlorosilanes led to, not the expected silicon compound analogous to a ketone, but to longchain polymers called silicones. • Silicones are water repellent because a silicone chain is surrounded by organic side groups and looks like an alkane from the outside. - Straight chain polymers of 20 -500 units are used as silicone fluids. - Silicone rubbers are made of long, straight chain polymers (dimethylpolysiloxanes) between 6000 and 600 000 Si units long, mixed with fillers normally finely divided SiO2 or occasionally graphite. • Uses of silicones: water repellents for treating masonry and buildings, glassware and fabrics. They are also included in car polish and shoe polish. Silicone fluids are non-toxic and have a low surface tension.