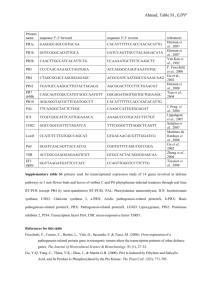
Functional Genomics of Tomato Interactions with Pseudomonas
advertisement

Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 Alan Collmer, PI, Cornell University James R. Alfano, coPI, University of Nevada at Las Vegas C. Robin Buell, coPI, The Institute for Genomics Research Samuel Cartinhour, coPI, Boyce Thompson Institute for Plant Research Arun K. Chatterjee, coPI, University of Missouri Terrence P. Delaney, coPI, Cornell University Sondra G. Lazarowitz, coPI, Cornell University Gregory B. Martin, coPI, Boyce Thompson Institute for Plant Research Xiaoyan Tang, coPI, Kansas State University Rapid progress has been made in our understanding of the molecular basis of plant-microbe interactions. Tomato has been important in that research because of its genetic tractability, its economic value, and the cost of the many diseases that afflict it. A centerpiece of that work has been the AvrPto-Pto mediated gene-for-gene interactions of Pseudomonas syringae pv tomato DC3000 and tomato. DC3000 has emerged as an important model in molecular plant pathology because of its genetic tractability, because it also is a pathogen of Arabidopsis, and because its interactions with plants appear representative of many prevalent bacterial and fungal pathogens. Consequently, national and international surveys among plant pathologists have yielded DC3000 as the first choice for the first phytopathogenic bacterium to be sequenced. An additional factor that makes DC3000 experimentally attractive is the evidence that its parasitic abilities are largely based on (an unknown number of) effector proteins (e.g., AvrPto) that are injected into plant cells by the Hrp (hypersensitive response and pathogenicity) type III secretion system. Identification of these effectors will provide the basis for orderly dissection of the molecular processes underlying pathogenicity, and these proteins, which presumably have evolved to exquisitely alter plant gene expression and metabolism, are a likely source of new tools for plant biologists. Consequently, the first objective of this proposal is the sequencing of the complete genome of DC3000 by The Institute for Genome Research (TIGR) and genome-based searches for Hrp effector genes (and other candidate virulence factors). Subsequent objectives involve microarray analyses to identify genes and regulons in both DC3000 and tomato that are likely to be important in the interaction and the development of a series of GFP-based tools for monitoring molecular/cellular events in living plant tissues during bacterium-plant interactions. Because DC3000 interactions with tomato (and Arabidopsis) involve highly localized cellular events and appear to be highly multifactorial and dependent on redundant factors, these new tools will be essential for a functional genomic analysis of pathogenesis and defense. Other important objectives of the proposal involve the establishment of a community-oriented functional genomics annotation web site and database that will serve the wider research community and also provide the centerpiece for educational outreach activities involving high school and undergraduate students. The specific objectives of the proposal are: 1. Characterize the P. s. tomato DC3000 genome: sequence, annotate, and compare with the genomes of P. aeruginosa and P. putida, and facilitate community access to sequence data in all stages of development and annotation. 2. Identify and characterize P. s. tomato Hrp effectors, novel virulence systems, and virulence regulators. 3. Characterize the interactions of tomato and P. s. tomato by microarray analysis, identify novel attack and defense regulons in both microbe and plant, develop novel bioinformatics-compatible cell biological assays and functional genomics tests. 4. Develop a community-oriented Pseudomonas-Plant Genome Interaction Database to support the functional genomics of interorganismal interactions and an educational outreach initiative involving development of a new high school level integrated science/math/social studies course on “Genomics: Applications and Implications.” PROJECT DESCRIPTION 1 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI Background The tomato-P. syringae pathosystem is broadly important in molecular plant pathology. Pseudomonas syringae pv tomato DC3000 (hereafter DC3000) causes bacterial speck disease in tomato and Arabidopsis (35, 179). Bacterial speck is an economically significant disease of tomato (97), but more importantly, the disease provides an ideal model for studying plant-pathogen interactions. Lessons gained from DC3000 are broadly applicable at several levels: (i) the pathogenically diverse strains comprising the species P. syringae collectively attack virtually all crop plants, (ii) P. syringae pathogenic mechanisms appear fundamentally similar to those of other common phytopathogenic bacteria in the genera Xanthomonas, Ralstonia, and Erwinia, and (iii) the host-specificity, disease symptoms, and plant defense responses associated with bacterial speck are similar to those associated with many important fungal pathogens (3, 145). As will be explained below, P. syringae parasitism of plants is directed by effector proteins that are injected into plant cells by bacteria colonizing the apoplast, and consequently, much of the pathogen recognition that underlies host range-limiting defenses occurs inside the plant cell. It now appears likely that the molecular battles controlling parasitism and host specificity of many fungal pathogens are similarly waged by proteins inside plant cells, as indicated by work with Magnaporthe grisea (rice blast) and Melampsora lini (flax rust) and their corresponding plant resistance genes (106, 168). We will highlight below the experimental resources available for the tomato-DC3000 pathosystem and the role of this pathosystem in the development of the injected-effector paradigm and other important concepts in molecular plant pathology. We will also show the relevance of this work to plant cell biology. Nevertheless, despite these advantages and advances, we still do not know for P. syringae or any of the myriad other host-specific bacterial and fungal pathogens what enables them to grow in hosts, what stops them in resistant plants, or what injected effector proteins are doing in host cells to promote parasitism. Furthermore, our understanding of the regulation of virulence is fragmentary, our knowledge of the intrinsic cellular processes in pathogenesis is based largely on tissue level assays, and our inventory of virulence-related genes is fundamentally incomplete because mutant screening assays largely fail with a process that is multifactorial and based on redundant factors. As explained below, new genomic and cell biological tools can efficiently remedy these deficiencies and enable the tomato-DC3000 pathosystem to provide a radically more comprehensive model of plant-pathogen interactions. Tomato is an economically important and experimentally tractable plant species. Tomato is a member of the Solanaceae family, a widely distributed group of plants to which many other crop species also belong, including potato, pepper, eggplant, tobacco, and Petunia. Economically, the Solanaceae is the third most valuable crop family in the U.S. and the most valuable family among vegetable crops. In addition to the major tomato production areas of California, Florida, New Jersey, and the Midwest, tomatoes are the most popular vegetable for home gardeners throughout the country. Tomatoes are a rich source of vitamin C and of lycopene, a compound with proven anti-oxidant ability that may lower the risk of certain cancers and heart disease. In addition to its economic and nutritional importance, several features make the tomato an excellent species for understanding basic plant biology. Tomato is diploid, has a small genome (950 Mb), a relatively short life-cycle, tolerates inbreeding, yet is easily crosshybridized, and is easy to grow and maintain. As a result of intensive breeding and genetics research with this species many resources are available including extensive germplasm collections, numerous natural, induced, and transgenic mutants and genetic variants, routine transformation technology, a dense RFLP map, numerous cDNA, YAC, BAC and other genomic libraries, and recently an extensive database of over 60,000 ESTs (www.tigr.org). Because of its experimental tractability, tomato has been the focus of many fundamental discoveries in plant biology, including control of gene expression by antisense/sense technology (70), transgenic analysis of genes which impact susceptibility or response to pathogen attack (72, 115), the discovery of a peptide hormone (systemin) (151), and the isolation of many disease resistance (R) genes (discussed below). Tomato is an excellent model for the study of plant disease resistance and susceptibility. Tomato is susceptible to a large number (>100) of diseases. Basic and applied research to minimize the impact of these diseases has resulted in the characterization of plant responses to numerous disease agents including bacteria, fungi, viruses, nematodes, chewing insects, and abiotic stresses. This knowledge, combined with the extensive germplasm collection of wild species of tomato, has resulted in the identification of more than 50 major disease resistance (R) genes as well as a number of QTLs controlling horizontal resistance (97, 165, 178). Tomato was the first plant from which a "gene-for-gene" class of R 2 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI gene was cloned - Pto (118) - and, in total, more R genes (nine) have been isolated from tomato than any other plant species. These include genes conferring resistance to fungi, nematodes, aphids, bacteria and viruses: Cf-2, (46); Cf-4, (163); Cf-5, (45); Cf-9, (96); I2, (128, 155); Mi/Meu1, (122), (144), (174); Pto, (118); Prf, (146); and Sw-5, (24). Additional R genes, some of which have been shown to function when transferred into tomato, have been isolated from related solanaceous species, including pepper, Bs2 (160); potato, Gpa2 (unpublished); and Rx (13); and tobacco, N (182). Significantly, many tomato R genes encode proteins that have unique features that have not been observed in R proteins from other plant species (17). For example, Pto consists of simply a protein kinase catalytic domain, Prf contains a large N-terminal region without homology to other known proteins, the Cf genes encode extracytoplasmic leucine-rich repeat proteins, and the Mi1/Meu1 gene encodes resistance to both nematodes and the potato aphid. In addition to yielding many R genes, tomato has been used to study various plant defense responses such as expression of "pathogenesis-related" genes, the oxidative burst, the role of systemin in insect resistance, and signaling events involving ethylene and jasmonic acid (127, 151, 167, 172, 183, 188). The many cloned R genes from tomato and the abundance of information and resources related to diverse plant defense responses provides an unparalleled foundation for increasing our understanding of plant responses to specific bacterial effector proteins as proposed here. P. syringae provides an excellent model for exploring the function of injected effector proteins in plant parasitism and host specificity. Strains in P. syringae are noted for their diverse and host-specific interactions with plants (82, 145). A specific strain may be assigned to one of at least 40 pathovars based on its host range among different plant species and then further assigned to a race based on differential interactions among cultivars of the host. Interactions with a single plant are also diverse. Infected seeds are an important source of primary inoculum in P. syringae diseases, and bacterial growth on leaf surfaces as an epiphyte often precedes disease development (81, 82, 145). The bacteria enter leaves through stomates, and then in host plants they typically grow to high population levels in intercellular spaces and produce necrotic lesions that are often surrounded by chlorotic halos caused by bacterial toxins. In nonhost plants or in host plants with race-specific resistance, the bacteria elicit the hypersensitive response (HR), a rapid, defense-associated programmed death of plant cells in contact with the pathogen (5, 139). The ability to produce either of these reactions in plants appears to be directed by hrp (HR and pathogenicity) and hrc (HR and conserved) genes that encode a type III protein secretion pathway and by avr (avirulence) and hop (Hrp-dependent outer protein) genes that encode effector proteins injected into plant cells by the pathway (5). These effectors may also betray the parasite to the HR-triggering R-gene surveillance system of potential hosts (hence the avr designation), and plant breeding for resistance based on such gene-for-gene (avr-R) interactions may produce complex combinations of races and differential cultivars (98). (Subsequent sections will provide more information on avr genes in general and then on avrPto and its interactions with the Pto R gene, specifically.) Screens for nonpathogenic P. syringae mutants typically yield hrp mutants (111, 112) and never avr mutants, which indicates the importance of the Hrp secretion system in pathogenesis and suggests redundancy in the effector gene system. hrp/hrc genes are probably universal among necrosis-causing gram-negative plant pathogens, and they have been sequenced in P. s. syringae 61, Erwinia amylovora Ea321, Xanthomonas campestris pv. vesicatoria 85-10, and Ralstonia solanacearum GMI1000 (5). The injection of effector proteins into host cells is an important ability of the type III secretion systems of both plant and animal pathogens (59). The evidence for this in plant pathogens, although indirect, is quite strong (5, 22), and it highlights the importance in parasitism of the collective set of effector proteins that are injected into plants. The Hrp system and its effector genes appear to have been horizontally acquired by P. syringae. The importance in bacterial evolution of horizontally acquired blocks of genes with related function is one of the major lessons of recent bacterial genome projects (107, 130). This is particularly important in the evolution of pathogenicity, which can occur in "quantum leaps" through the acquisition of "pathogenicity islands" (67, 68). The Collmer and Alfano labs have sequenced the 50-kb Hrp pathogenicity island of DC3000 and compared it with the Hrp pathogenicity islands of P. s. syringae strains 61 and B728a (4). Strain 61 is a weak pathogen of bean whose cloned hrp/hrc/hopPsyA gene cluster has been extensively studied because it directs saprophytes like P. fluorescens and E. coli to elicit the HR in tobacco (43, 78, 84, 85, 86, 87, 191). The hrp/hrc gene cluster is conserved in all three strains and is flanked on the left by a unique exchangeable effector locus and on the right by a conserved effector locus. The exchangeable 3 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI effector locus contains mobile genetic elements and from 1 to 4 avr/hop genes that are different even in strains of the same pathovar. The conserved effector locus carries at least 7 ORFs that are conserved in the divergent pathovars syringae and tomato. We have deleted the entire hrp/hrc gene cluster from DC3000 and complemented the deletion with the corresponding genes from P. s. syringae 61 (32). As expected, the DC3000 hrp/hrc deletion mutant causes no visible reaction in plants, but the mutant complemented with the Hrp system from the bean pathogen still elicits the HR in bean and multiplies and causes speck lesions in tomato (32). Thus, host specificity is determined by the effectors, the Hrp system appears to function universally among P. syringae pathovars, and lessons learned about secretion mechanisms from the model Hrp system of P. s. syringae strain 61 should be directly transferable to DC3000. avr genes appear highly mobile, and Avr proteins represent a menagerie with respect to sequence and physical properties. Over thirty avr genes have been cloned from various strains of P. syringae and X. campestris on the basis of their avirulence phenotype (109, 173). Whereas the avirulence phenotype is strong (R gene-dependent HR elicitation), the virulence phenotypes of these proteins is typically weak (quantitatively reduced growth in compatible host plants). The potential horizontal transfer of avr genes into and among P. syringae strains and other plant pathogenic bacteria is supported by their frequent linkage with transposable element and bacteriophage sequences (73, 100), their relatively low %GC content (173), the appearance of some in exchangeable effector loci, and the functional interchangeability of the E. amylovora dspE and P. s. tomato avrE genes (21). The relatively weak contribution of individual P. syringae avr genes to parasitic fitness is presumably the result of long coevolution with plants, wherein mutations in plant susceptibility targets that would diminish parasitic benefit and R gene surveillance are two factors that could promote effector protein redundancy. Nevertheless, a primary role for "Avr" proteins in virulence seems certain, although the nature of that role is still a puzzle with relatively few clues. AvrRxv from X. campestris pv. vesicatoria has a homolog among the effector proteins of animal pathogens, namely YopJ/P of Yersinia and AvrA of Salmonella, with YopJ being a MAP kinase inhibitor that suppresses macrophage defenses (74, 129). AvrBs2 shows similarity with agrocinopine synthase (opine production in tumors) of Agrobacterium tumefaciens, which suggests a direct role in the nutrition of the pathogen (159). AvrBs3 family members, which are widespread in pathogenic Xanthomonas spp., appear to be transcription factors (198). There is also evidence that Hrp-secreted factors (and particular avr gene products) may suppress plant defenses (94, 95, 176). Experiments involving heterologous expression of avr genes inside plant cells suggest that Avr proteins can be deleterious even in the absence of a known cognate R gene if expressed too strongly (23, 63, 120), and pthA (avrBs3 family) elicits several disease-like symptoms when transiently expressed in citrus cells (48). Whether these effects result from interaction with susceptibility targets in the host is unknown. Finally, it is important to note that some Avr proteins, such as members of the X. campestris AvrBs3 family, carry nuclear localization signals and are targeted to the plant cell nucleus, and others, such as P. syringae AvrRpm1 and AvrB (and possibly AvrPto) carry N-terminal myristylation sites and may be myristylated and then localized to the inner face of the plasma membrane upon delivery to plant cells (23). The phytotoxin coronatine is one of several other known P. s. tomato virulence factors. Coronatine is comprised of polyketide and isoleucine-derived structural components, and it partially mimics methyl jasmonate, a growth and defense regulator derived from the octadecanoid signaling pathway (15). Coronatine causes diffuse chlorosis around lesions, plant cell wall thickening, and changes in chloroplast structure in tomato (131), and it suppresses defense gene expression in Arabidopsis (123). Coronatine contributes to the number and size of bacterial speck lesions in tomato (16, 93, 123). Other factors affecting parasitic fitness or symptom production that have been identified in various P. syringae pathovars include the exopolysaccharide alginate (194), pathogen-produced auxin (62, 119), type IV pili (142, 157), flagella (77, 132), and pectate lyase (11). There are many gaps in our knowledge of the regulation of P. syringae virulence systems. The global regulation of virulence in nontumorigenic, phytopathogenic bacteria has been particularly well studied in E. chrysanthemi, E. carotovora, E. stewartii, R. solanacearum, and X. campestris (29, 47, 88, 90, 170, 180). In contrast, our knowledge of virulence regulation in P. syringae is more limited to individual virulence systems. Thus, we know that coronatine production in various P. syringae strains is induced by the cool temperatures (18-22 C) that favor disease (141) and by plant organic acids (110), 4 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI and that the phytotoxin syringomycin is induced in P. s. syringae by host phenolic glucosides (124). Similarly, the Hrp system is activated in apoplast-mimicking minimal media by a regulator cascade that includes HrpR and HrpS (NtrC family regulators) and HrpL, an alternative sigma factor of the ECF (extracytoplasmic factor) family. HrpL activates the transcription of hrp/hrc and avr genes in P. syringae, which carry the "Hrp box" 5'-GGAACCNA-N(14)-CCACNNA-3' (65, 66, 91, 92, 154, 189, 190). HrpV, a negative regulator of hrp gene expression was recently identified (136), but little is known about its function or about other factors that act upstream of HrpR/S. A notable exception regarding the paucity of known global regulators in P. syringae is the two-component GacA/GacS system, which in pathovar syringae controls the production of syringomycin, protease, N-acyl homoserine lactone, and disease symptoms, but not bacterial growth in hosts or elicitation of the HR in nonhosts (49, 83, 102, 184, 185). Because GacA/GacS (and downstream regulator SalA) activate genes required for lesion formation but not parasitic growth, whereas HrpL activates genes required for symptomless parasitism, a genomic analysis of these two regulons would be particularly informative. The interaction of the tomato Pto and bacterial AvrPto proteins initiates resistance to bacterial speck disease and is a model system for understanding the molecular basis of "gene-for-gene" disease resistance. The two races of P. s. tomato known are distinguished by either the presence (race 0) or absence (race 1) of the effector gene avrPto. The avrPto gene was cloned in the early 1990s (143) and its protein was recently shown to be secreted from DC3000 (which is race 0) by the Hrp secretion system (169). The Pto gene was isolated by map-based cloning and shown to encode a small serine/threonine protein kinase (118). Subsequently, the AvrPto protein was shown to function inside of the tomato cell by interacting directly with the Pto kinase (152, 161) and a single threonine residue of Pto was found to determine its recognition specificity for the avirulence protein (57). Thus, the molecular basis of recognition specificity in this "gene-for-gene" interaction lies in the interaction of a host resistance protein and a bacterial effector protein. The recent characterization of several other genes involved in Pto-mediated resistance further enhances the potential of this system for understanding plant resistance to bacterial diseases. The Prf gene (Pseudomonas resistance and fenthion sensitivity) was identified by using a map-based cloning strategy and encodes a protein with similarity to other leucine-rich-repeat R genes (Salmeron et al., 1996). The Martin laboratory has isolated the Pti1 gene (Pto-interacting) which encodes a protein kinase that is phosphorylated by Pto and three genes, Pti4, Pti5, and Pti6, that encode transcription factors which interact with the Pto kinase and bind to a cis element in the promoters of many pathogenesis-related (PR) genes (196, 197). Based on this work, the Martin laboratory has proposed a model in which the physical interaction of Pto and AvrPto (possibly also involving the Prf protein) activates the Pto kinase. Activated Pto then phosphorylates and activates diverse downstream target proteins, each with a unique role in the resistance response. The Pti1 kinase is one such target and is involved in the HR leading to localized cell death. The Pti4/5/6 transcription factors are involved in a separate pathway leading to activation of certain PR genes. Ultimately, disease resistance is determined by the activation of a variety of defense responses including the oxidative burst, nitric oxide production, defense gene expression, and the hypersensitive response (42, 50, 71). Many aspects of bacterium-plant interactions are still puzzling, and they point to the need for cell biological assays. Although a model centered on Hrp-injected effector proteins provides a robust framework for formulating further hypotheses, it ignores a number of exceptions and gaps in our growing picture of bacterial pathogenesis. It is possible that some Avr/Hop effector proteins will be found to act outside of plant cells, as suggested by the finding that the rice Xa21 R gene has an extracellular leucinerich repeat domain (156). Other Avrs, such as AvrD, may operate inside bacterial cells by producing low molecular-weight elicitors of R gene-mediated plant defense (and presumably parasitic benefit in some plants)(99). The cell death that marks the HR is not essential for R gene-mediated resistance against P. syringae (193), and different R-genes may impinge on different signaling pathways, based on studies of mutants and on the profile of induced responses (1). There is a bewildering array of defense responses, and many of these may be localized to the region of the plant cell surface that is in contact with the pathogen (18, 25, 192). This limits the interpretation of assays based on defense expression and bacterial multiplication at the tissue-level, and the majority of known P. syringae effector genes have no virulence phenotype in such assays. In summary, bioassays capable of discerning the molecular and cytological interactions of individual host and pathogen cells will be required to dissect the molecular basis of DC3000-tomato interactions. 5 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI Preliminary Results and Biological Resources Available A growing set of tools are available for identifying and characterizing novel P. syringae effector proteins. The potential to be targeted to the Hrp pathway is one property that all Hrp effector proteins can be predicted to share, and it is the only property that can be used to identify such proteins in culture without plant bioassays. To exploit this property, the Collmer and Alfano labs have investigated Hrp secretion mechanisms and targeting signals. We have developed a universal system for identifying P. syringae Hrp secretion substrates using the E. chrysanthemi EC16 Hrp system cloned in E. coli (69). We also have determined the optimum pH and temperature conditions for secretion of Avr/Hop proteins from P. syringae and determined that AvrPto (and probably AvrB) use a universal mRNA targeting signal that is also recognized by the type III secretion system of Yersinia (8, 169). In a separately funded NSF project, we are continuing to improve the technology for characterizing Hrp secretion substrates and their injection into plant cells through the use of reporter fusions, hrp mutants, and various translocation assays. A common feature of type III secretion systems is host cell contact-dependent activation (59). In investigating this possibility with the aid of a plasmid-borne PhrpA::gusA fusion and antibodies against various Hrp proteins, we have learned that the addition of suspension-cultured tobacco cells to bacteria in medium that mimicking apoplastic fluids stimulates further hrp gene expression ca. 20-fold. Our ongoing search for bacterial mutants and plant factors that may promote this fully induced level of hrp expression should provide more tools useful for this project. A bioassay based on GFP-labeled DC3000 has revealed a novel phenotype that is the prototype for a new class of assays. The virulence phenotypes of P. syringae avr/hop mutants have been typically determined by plate counts of bacterial populations in infected leaf samples. This method detects only relatively large changes in the parasitic fitness of mutants. For example, it reveals only a statistically insignificant reduction in the growth of a DC3000 mutant deficient in hopPtoA (a gene in the Hrp pathogenicity island conserved effector locus that encodes a Hrp-secreted protein and potential effector). By transforming DC3000 wild-type and hopPtoA mutants with pTB93F, which constitutively expresses green fluorescent protein (GFP) (58), and then developing methods for measuring bacterial colony sizes in undisturbed Arabidopsis and tomato leaf tissue by laser-scanning confocal fluorescence microscopy, we have learned that HopPtoA contributes specifically to the initiation of colony growth on the surface of mesophyll cells. That is, significantly more of the hopPtoA mutants remain as single cells in planta, but those that do initiate growth produce colonies as large as the wild type. We now can use this assay to quantitatively determine the effects of any bacterial or plant gene on any stage of bacterial colony development, and we can precisely correlate changes in colony phenotype with changes in plant gene expression and symptom expression. AvrPto has a detectable virulence phenotype that is controlled by a domain not involved in Pto interaction. In the Tang lab we have found recently that avrPto not only has an avirulence function, it also has a virulence activity. When avrPto is introduced into the P. s. tomato race 1 strain T1, it significantly increases bacterial growth and disease symptoms in tomato plants lacking Pto. Similar to avrA and avrE (114), addition or deletion of avrPto did not affect the virulence of DC3000, a P. s. tomato strain that is more virulent than T1. Four point mutations in avrPto were identified using a random mutagenesis approach that disrupted the avirulence but not the virulence activity. These mutations are tightly clustered between Ser94 to Gly99 of AvrPto and likely define a motif specific for avirulence. Studying the in planta function of AvrPto has been difficult because uncontrolled expression of AvrPto is deleterious to plant development. We employed a tetracycline-inducible gene expression system (61) to express AvrPto in transgenic plants (Tang et al., unpublished). Using this method, we found that the AvrPto protein was exclusively associated with the plant cell membrane. AvrPto is a hydrophilic protein, but it carries a conserved myristylation motif (Met-Gly-x-x-x-Val-) at the N-terminus of the protein that may be responsible for its membrane localization. We examined the functional relevance of this motif by replacing Gly2 (GGA) with Ala (GCA). When introduced into P. s. tomato strain T1, the G2A mutant was no longer avirulent in plants expressing Pto and lost its virulence activity in plants lacking Pto. The loss of function was not due to the diminished secretion of AvrPto, because T1 strains carrying the G2A mutant and the wild type avrPto secreted the same amount of AvrPto protein into the inducing medium (Tang et al., unpublished). Consistent with this, Agrobacterium-mediated transient expression of the G2A mutant 6 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI failed to induce hypersensitive cell death in Pto-expressing plants. Finally, a yeast two-hybrid assay demonstrated that the G2A mutant interacted normally with Pto. We have developed a DC3000 yeast two-hybrid "prey" library and isolated a candidate DC3000 effector protein that interacts with Pto. Recently, the Martin laboratory has developed a yeast twohybrid approach to identify DC3000 effector proteins (in addition to AvrPto) that interact with the Pto kinase. The rationale for these experiments comes from the original experiments to characterize avrPto in which a gene replacement strategy was used to delete the avrPto gene from DC3000 (143). Interestingly, the avrPto DC3000 strain was still recognized by a tomato line carrying the Pto locus (143). In following up this observation, we have found that a tomato line carrying a 35S::Pto transgene is also resistant to the avrPto DC3000 strain (Martin, unpublished). These results indicate that DC3000 carries additional effector proteins that are recognized specifically by the Pto kinase. Because the Pto kinase physically interacts with AvrPto, it seemed possible that other DC3000 effector proteins might also physically interact with Pto. In order to identify such proteins, twelve genomic libraries (3 vectors x 4 restriction enzymes) have been constructed in a modified series of yeast two-hybrid "prey" vectors. Each of the three vectors has its cloning site in a different reading frame in order to maximize the chance of cloning a portion of all DC3000 ORFs as functional fusions with the Gal4 transcriptional activation domain. Four different restriction enzymes were used to maximize the coverage of the genome. A screen of these libraries using Pto as a bait identified three avrPto clones and several clones that encode other candidate effectors. One class, for which 8 clones were retrieved, has been shown to have a 1.6 kb ORF with a typical Hrp box upstream. A representative of this class, now termed "AvrPto2", interacts in an identical fashion as AvrPto with a large series of Pto chimeric (and mutant) proteins that were previously described (57). Experiments are underway to characterize the potential avirulence (or virulence) activity of AvrPto2. A key question we are addressing is whether a DC3000 avrPto/avrPto2 mutant will now cause disease rather than the HR in Rio Grande Pto-R. Although the DC3000 genomic libraries were developed for twohybrid screening against Pto they are also useful for many other purposes such as development of DC3000 microarrays. We have made substantial progress in development of an EST database and cDNA microarrays for tomato. As part of an ongoing NSF Plant Genomics grant (DBI-9872617) the Martin lab is developing tomato cDNA microarrays for gene expression profiling of plant response to various pathogens and elicitors. This project involves: (i) development of cDNA libraries derived from tomato leaves undergoing defense responses; (ii) sequencing of large numbers of these cDNAs and the establishment of an EST database (by TIGR); (iii) derivation of a "unigene" set consisting of one representative cDNA of each unique tomato gene; and (iv) development of cDNA microarrays for expression profiling. The three libraries developed to date and the number of ESTs deposited in GenBank (as of 12/20/99) are: cLER (Pseudomonas resistance response), current ESTs: 5,399; cLES (Pseudomonas susceptible response), current ESTs: 5,966; cLET (mixed elicitors), current ESTs: 10,349. These ESTs, along with an additional 40,000 sequences derived from other cDNA libraries have been used to derive a set of 8,000 unigenes. To date, 5,000 of these clones have been rearrayed into 384-well microtiter plates and their inserts are being PCR amplified for microarraying onto glass slides. The microarraying is being done in the new Center for Gene Expression Profiling that was established in the past year at the Boyce Thompson Institute (see Facilities description). PCR products (approx. 4 nl each) are spotted at a 200 micron pitch onto silanated glass slides with a Genetic Microsystems arrayer. As controls for hybridization and for standardizing among slides the following genes are replicated several times on each slide: rubisco, osmotin, ubiquitin, actin, several non-plant genes, and the pBluescript polylinker. For labeling, poly A+ RNA is first purified from total leaf RNA using the Promega PolyAttract system. A first-strand reverse transcription reaction is then performed with poly A+ RNA, dNTPs and Cy3-dUTP (or Cy5-dUTP). Unincorporated Cy3/5 is removed with a Centricon-30 spin column and the probe is resuspended in 10 l of 3X SSC / 0.3% SDS. Hybridization is done overnight at 65oC in enclosed plastic cassettes (Telechem). After hybridization, the slide is washed for 5 min in 1X SSC / 0.1% SDS, 5 min in 0.1X SSC / 0.1% SDS, 2 min in 0.1X SSC. Using this procedure we have made and analyzed prototype slides that contain 4,096 DNA spots (64 x 64 grid). We are continuing to optimize the procedure further in preparation for spotting the nonredundant clone set of 8,000 elements. The first phase of the ongoing work will focus on gene expression profiling of tomato leaves over a time course in response to DC3000 with (or without) avrPto and avrPto2. In the future, the analyses will be extended to tomato leaf responses to other pathogens 7 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI (e.g. Phytopthora infestans, Xanthomonas vesicatoria, tomato spotted wilt virus) and to various elicitors (e.g. salicylic acid, jasmonic acid, ethylene, fenthion). These studies, while not overlapping with the experiments in the present proposal, will lead to the development of an extensive database on tomato defense gene expression that will be useful for comparison with the regulons we define as responding to specific DC3000 mutants and effector proteins. Rationale and Relevance We are proposing a functional genomics analysis of the interactions of P. s. tomato DC3000 and tomato that will be based on the complete genome sequence of the pathogen, the subset of plant genes that are induced during interactions with the pathogen, and a set of bioinformatics-compatible cell biological assays that will aid genetic dissection of pathogenic processes. The sequencing of the DC3000 genome is central to this proposal, and it will have a significant impact on plant biology, molecular plant pathology, and pathogenic microbiology. Specifically, the genomic sequence will yield (i) a global picture of the adaptations of a single, highly coevolved parasite that can be simultaneously applied to studies with tomato and Arabidopsis, (ii) a complete inventory of effector proteins, toxins, and other factors with which the pathogen subverts host signal transduction and metabolism (a likely source of new tools for plant biologists), (iii) new insights into mechanisms of plant susceptibility and defense (and consequent new disease controls) that are likely to be relevant to understanding plant responses to a broad range of bacterial and fungal pathogens, and (iv) insights into the evolution of pathogenicity and metabolic diversity issuing from comparisons with the closely related bacteria, P. aeruginosa PAO1 (an animal pathogen), and P. putida (a soil bioremediation agent). Indeed, given the unique status of DC3000 as a well-studied, genetically tractable pathogen of two model plants, it is not surprising that surveys conducted in association with the American Phytopathological Society and the International Congress of Plant Pathology have yielded DC3000 as the top priority for bacterial plant pathogen genome sequencing. Furthermore, the DC3000 genome database will provide the basis for a variety of educational outreach activities that will be uniquely able to link genomics, agriculture, evolution, disease, and the associated dynamics of interorganismal interactions. The functional genomics portion of the proposal is directed toward enhancing our understanding of both the host and microbial responses that underlie parasitism, symptom production, and defense. Our goals are guided by several working hypotheses and resultant strategies: (i) Hrp effector proteins are likely to be particularly important in DC3000 pathogenesis, therefore, identifying these proteins and determining their effects on the plant will be a key part of the project. (ii) Some Hrp effectors are likely to act as highly localized suppressors of plant defenses and remodelers of plant cell structure, whereas bacterial toxins and other virulence factors may promote parasitism through more diffuse effects on host tissues, and host responses will have similarly differing degrees of localization; therefore the development of molecular/cell biological assays for plant responses to pathogen mutants and isolated effectors and a spatial-temporal model of pathogenic processes must be central to the project. (iii) The products of perhaps 200 genes that are differentially expressed in each organism during the interaction are likely to be particularly important in determining the outcome (in addition to constitutively expressed defense surveillance factors); therefore identifying these genes will be important, and these genes will form the highly annotated core of the Pseudomonas-Plant Genome Interaction Database. (iv) Pathogen and plant genes operating at a particular stage of attack or defense are likely to be part of the same regulon; therefore identifying and manipulating key regulators of these regulons will provide the most efficient way to assess the role of groups of factors with related function. (v) The collection of interaction-specific genes that we will identify is likely to reveal a variety of novel factors; therefore we will be open to significant revision of our general model of the plant-pathogen interaction, and we will be prepared to evaluate the role of these novel factors. (vi) The interaction of P. syringae and plants is highly multifactorial; therefore, each of the cell biological assays that form the basis of our functional genomics analysis must be bioinformatics compatible. That is, standardized inoculation procedures, internal standards, and quantitative observations will enable equivalent results to be obtained in any lab and then entered into appropriate fields in the database. This will facilitate analysis of the relative role of multiple factors contributing, for example, to the initiation of bacterial colony development in planta. The proposal is highly distributive with regard to resources and responsibilities among the nine coPIs and is designed to efficiently generate a more comprehensive model of DC3000-tomato interactions. This model will comprise a new set of testable hypotheses that will spawn further, independently funded projects in many labs, including those of the coPIs. Although the limited resources of this proposal will 8 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI leave undone many important long-term objectives, such as yeast 2-hybrid searches for plant proteins interacting with various bacterial effectors, we feel that our highly distributed genomics approach will move the field ahead most rapidly. To foster the efficient development of the Pseudomonas-Plant Genome Interaction Database, all members of the community will be invited to contribute their expertise in the annotation, according to the model of the P. aeruginosa Community Annotation Project. For example, Carol Bender has agreed to annotate the genes directing the biosynthesis of coronatine and extracellular polysaccharides, and Bob Hancock will bring his extensive experience with P. aeruginosa and Helicobacter outer membrane protein genes to the annotation of the corresponding DC3000 genes. We expect that additional biological annotation will result from the stimulating effect of the DC3000 genome sequence on the ongoing research of several labs, including Steve Lindow's on the genetics of epiphytic fitness in P. syringae and Sheng Yang He's on three unidentified proteins that are secreted by the Hrp system of DC3000 in culture. There is also a dynamic community of researchers using DC3000 as a model pathogen of Arabidopsis, and we will design the Pseudomonas-Plant Genome Interaction Database to accommodate functional genomics data issuing from that pathosystem. Specific Objectives 1. Characterize the P. s. tomato DC3000 genome: sequence, annotate, and compare with the genomes of P. aeruginosa and P. putida, and facilitate community access to sequence data in all stages of development and annotation. 2. Identify and characterize P. s. tomato Hrp effectors, novel virulence systems, and virulence regulators. 3. Characterize the interactions of tomato and P. s. tomato by microarray analysis, identify novel attack and defense regulons in both microbe and plant, develop novel bioinformatics-compatible cell biological assays and functional genomics tests. 4. Develop a community-oriented Pseudomonas-Plant Genome Interaction Database to support the functional genomics of interorganismal interactions and an educational outreach initiative involving development of a new high school level integrated science/math/social studies course on “Genomics: Applications and Implications.” Experimental Plan Overview The work plan begins with the pathogen genome sequence and several sequence- and microarraybased searches for host-pathogen interaction genes. The searches are expected to yield perhaps 200 genes in each partner that may have some role in the interaction. Second generation microarrays of plant and bacterial interaction genes will then be constructed. Interactions between plant and pathogen will be dissected with bacterial mutants, isolated Hrp effector proteins, and a limited set of defense-altered transgenic tomato lines. Changes in the interaction will be monitored with the second generation microarrays and with novel GFP-based assays involving reporter bacteria and cell biological indicator plants. Microarray and functional genomics results will form the core of a community Pseudomonas-Plant Genome Interaction Database. We plan for initial interaction assays and microarray analyses to be based on tomato Rio Grande Pto-R plants inoculated with wild-type DC3000 (HR), DC3000 avrPto/avrPto2 (disease), and DC3000 hrp/hrc (nonpathogen-like null reaction). (Testing our expectation that DC3000 avrPto/avrPto2 will possess nearly full virulence on RG Pto-R is now being done as part of the preliminary studies.) The Pto-dependent resistant reaction will provide a benchmark in this work, but our emphasis will be on the genome-wide analysis of differences between disease and null interactions (and the underlying factors), which should reveal how bacteria become parasites and how plants become susceptible. Most of the plant pathological and molecular biological procedures referred to in this proposal are published and/or in active use in our labs, and we will provide details only for technically novel approaches. The appended Management Plan will explain the distribution of effort. 1. Characterize the DC3000 genome: sequence, annotate, and compare with the genomes of P. aeruginosa and P. putida, and facilitate community access to sequence data in all stages of development and annotation. We propose to use a shotgun sequencing approach to generate the complete sequence of the ~ 6 Mb genome of DC3000. TIGR has optimized this method, as reflected in the 10 published microbial genomes (27, 53, 54, 55, 56, 60, 103, 125, 166, 181) and the 21 current microbial sequencing projects at TIGR (www.tigr.org/tdb). One of the microorganisms TIGR is sequencing is the fluorescent pseudomonad, P. putida, which is closely related to P. s. tomato. Thus, the sequencing 9 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI and closure methodologies, as well as the annotation experience, that we have gained from our P. putida genome sequencing project, can be leveraged to our P. s. tomato genome project. For example, issues such as high G/C content and its impact on standard sequencing methodologies have already been addressed at TIGR with P. putida. 1a. Shotgun sequencing DC3000. The precise genome size of DC3000 is not known. However, based on the genome size of related pseudomonads, such as P. aeruginosa (5.9 Mb; www.pseudomonas.com), P. putida (6.1 Mb; K. Nelson, TIGR, pers. comm.), P. s. phaseolicola (5.64 Mb)(36), and P. s. ribicola (5.55 Mb)(28), we anticipate P.s. tomato DC3000 to have a genome size of 5.5-6 Mb. Two plasmids are known to be present in DC3000 and are estimated to be 65-79 kbp (35). High molecular weight DNA (genomic and plasmid) will be isolated from DC3000 using standard DNA isolation procedures (9). The DNA will be nebulized, ligated to BstXI adaptors, and size-selected on an agarose gel. Two shotgun libraries will be constructed in the modified pBR322 vector, pHOS2. (Note, that although a DC3000 library has been constructed in pJG4-5, as described above, TIGR has optimized sequencing methodologies for in-house modified vector pHOS2, and construction of the library for high throughput sequencing is a minor component in the overall costs to sequence a microbial genome.) We will construct a small insert library of 2-3 kbp and a larger insert library of 8-10 kbp. A small number of clones (n=24) will be tested via PCR amplification to verify the size of the inserts. Subsequently, 96 clones will be sequenced to assess the randomness of the library and efficiency of sequencing success. All templates will be prepared from the shotgun clones using the Qiagen 96 Real kit. A Q-bot that automatically picks colonies from plates and an automated template prep and quantification robot are being tested by the TIGR Research and Development team, and once these robotics are up to TIGR standards, they will be incorporated into the template production pipeline. Sequencing reactions will be performed using primarily BigDye Terminators (Perkin-Elmer, Applied Biosystems, Foster City, CA) using reaction conditions optimized for TIGR’s high throughput sequencing pipeline. Alternative sequencing chemistries will include Dichlororhodamine terminators and Big Dye Primers (Perkin-Elmer, Applied Biosystems, Foster City, CA). Use of ET chemistry (Amersham Pharmacia Biotech, Piscataway NJ) has been extremely successful in resolving sequences within stretches of G/Cs in TIGR’s P. putida genome project and we will test this alternative chemistry for its efficacy with the DC3000 genome. Sequencing reactions will be run primarily on ABI 3700 sequencers (Perkin-Elmer, Applied Biosystems, Foster City, CA) and when necessary on ABI 377 sequencers. We will sequence the small insert library to ~6-fold coverage and the large insert library to 2-fold. In several microbial sequencing projects at TIGR (W. Nierman, TIGR, pers. comm.), this ratio of coverage results in better assemblies of the random sequences, such that subsequent closure steps are greatly reduced. As we are not changing our overall fold of coverage by sequencing from two libraries, we have the option to change the ratio of large insert to small insert sequences during the grant period. Thus, if new data indicates we should increase the ratio of sequences from the large insert library, we can do so without changing our overall coverage. In addition, a bacterial artificial chromosome (BAC) library can also be constructed and through end sequencing of a relatively small number of clones, a scaffold composed of BAC clones can be for assembled for the genome. During the random sequencing phase we will assemble the DC3000 genome at 1X, 3X, 5X, and 8X coverage using the TIGR Assembler program (158). This will provide data on the randomness of the two libraries, the ability of the large insert and BAC end sequences to provide a scaffold for assembly of the entire genome, and an opportunity to preview the repeats in DC3000. Once the DC3000 genome has reached 8-fold coverage, it will be removed from the random sequencing stage and closure techniques will be used to bring the genome to closure. Molecular techniques used in closure include construction of microlibraries, transposon mutagenesis of selected clones, alteration of sequencing chemistries, and multiplex PCR. Numerous software tools are utilized at TIGR for closure. These include identification of failed mates, automated primer design to close sequencing gaps, as well as modification of the TIGR GROUPER program that groups assemblies to accommodate multiple libraries used in random sequencing. TIGR has enhanced the approach to closure of a microbial genome and we estimate that the ~6 Mb DC3000 genome will take 6-9 months to close. 1b. Primary annotation of the DC3000 genome. We will annotate the DC3000 genome using tools that have been well-established with other microbial annotation projects at TIGR (27, 53, 54, 55, 56, 60, 103, 125, 166, 181). Annotation will involve prediction of open reading frames (ORFs), sequence 10 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI similarity searches with nucleic acid and protein databases, and assignment of all the putative genes into role categories that reflect biological function. For ORF predictions, we will use the GLIMMER algorithm (147) that utilizes interpolated Markov models for gene identification. Database searches will be performed using an initial BLASTP search, followed by REPRAZE, a modified Smith-Waterman algorithm (177). Another strategy for identifying homology to known sequences is searching the ORFs against both the Pfam database (10) and an internal TIGR database of hidden Markov models (HMMs). We will further characterize the ORFs in the DC3000 genome to identify motifs in the predicted proteins. This will include identification of membrane-spanning regions using TopPred (31) and identification of signal peptides using Signal-P (126). tRNAs will be identified using the program tRNASCAN-SE (51). Other RNAs, ribosomal and structural, will be identified by homology to the known RNAs from other organisms. Each ORF will be assigned to one of 112 role categories (modified from (140)) based on the putative function of the protein as reflected by the sequence similarity and motif identifications as described above. Although the primary annotation of DC3000 will be done at TIGR, the collaborators on this project and the larger P. syringae community will also be involved in contributing expertise to the annotation. Coordination of the annotation among the collaborators will initially involve electronic correspondence and if needed TIGR can host the collaborators at the final stages of annotation. 1c. Comparative analyses of three Pseudomonas genomes. The completion of the DC3000 genome will be the third complete genome of a fluorescent pseudomonad, as the genomes of two Pseudomonas species, P. putida (K. Nelson, TIGR, pers. comm.) and P. aeruginosa (www.pseudomonas.com) will be complete prior to the start of this grant. All three species share similar biochemical and physiological properties yet are capable of inhabiting multiple ecological niches. P. aeruginosa is an opportunistic animal pathogen, although some isolates of P. aeruginosa are capable causing diseases on plants (138). In contrast, P. putida is a saprophytic, soil-inhabiting bacterium that can be found in close association with plant roots (164). The genome of P. s. tomato will be compared to the P. putida and P. aeruginosa genomes using the large-scale alignment system MUMmer (41) developed by several TIGR researchers. This will allow us to identify syntenic regions, large-scale genome rearrangements and reversals, and to map single nucleotide polymorphisms. As with the annotation of DC3000, TIGR will coordinate comparative analyses between these Pseudomonas species with collaborators who have extensive knowledge regarding Pseudomonas virulence and pathogenicity mechanisms, which will be a focal point in our comparative analyses. 1d. Data will be released rapidly by TIGR to the scientific community. As described in more detail in the appendix, the sequence of the DC3000 genome will be available to academic, not-for-profit researchers through a licensing agreement at the 3X, 5X, and end of random (~8X) stages of random sequencing (http://www.tigr.org/BlastSearch/License_UnfinishedGenomicData.html). The unfinished genome will also be available for BLAST searches on the TIGR web site (http://www.tigr.org/cgibin/BlastSearch/blast.cgi?). Annotation of DC3000 will be made available upon completion of annotation through publication in a peer-reviewed journal and through the TIGR web site (http://www.tigr.org/tdb). 2. Identify and begin characterization of DC3000 Hrp effectors, novel virulence systems, and virulence regulators. In this objective we will use three approaches to identify all of the DC3000 effector genes that are part of the Hrp regulon, and we will use both bioinformatic and microarray analysis in culture to begin the process of identifying novel virulence systems and key regulators of virulence systems. Finally, we will begin characterization of candidate effector genes, and we will begin construction of DC3000 mutants with deficiencies in effector genes, candidate virulence genes, and potentially key regulatory genes. In objective 3 the microarray-based analysis of novel effectors and other virulence genes will be extended to those genes that may be specifically induced in association with plants. 2a. Identify candidate effector genes outside of the hrp/hrc gene cluster by reporter transposon tagging of HrpL-activated genes. A potentially useful characteristic of known avr/hop effector genes in P. syringae is their HrpL-dependent expression (91, 92, 154, 190). We have already begun exploiting this to identify additional effector genes by Tn5gusA7-tagging in a DC3000 derivative in which the hrp/hrc cluster has been deleted and then partially complemented with a hrpL gene (from P. s. syringae 61) that is expressed from the tac promoter of vector pVLT35 (37, 153). Deletion of the hrp/hrc 11 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI cluster eliminates the background of positive hits in non-effector genes. An initial screen of 3000 mutants has yielded 7 colonies that are reproducibly blue in an IPTG-dependent manner on plates containing Xgluc and thus involve potential effector genes. The transposon and flanking sequences have been cloned and are now being sequenced. These experiments are expected to yield several effector genes and corresponding mutants for use early in the project, and the 5' sequences will yield additional functional Hrp boxes, which will help us refine the genomic queries proposed in the next objective. 2b. Identify candidate effector genes and other potential virulence factors by searching DC3000 genomic sequences for Hrp boxes. The initial annotation will group many of the identified ORFs into categories that predict their product’s biochemical function. For example, one large group would be genes that are involved in DNA repair and synthesis. Another large group will likely be a set of ORFs that have no predicted function based on obvious identity with known proteins in the databases. This category will be analyzed first for the presence of Hrp boxes. Since known effector genes are preceded by Hrp boxes, searches for this motif using a variety of sequence analysis programs (e.g., DNAStar) will provide an efficient means to identify effector genes in all stages of the sequencing project. 2c. Identify candidate effector genes and other potential virulence factors by microarray analysis of genes activated by HrpL in culture. The microarray analysis will be based on a comparison of wild-type DC3000 and hrpL mutant strains grown in apoplast-mimicking medium at 18-20oC (91). Initially, twelve DC3000 genomic libraries constructed in a yeast two-hybrid vector series (see Preliminary Data) will be used to develop microarrays. Considering the DC3000 genome to be 6 Mb, and the average insert size of the genomic libraries to be 1 kb, then a set of 18,000 clones will contain 3x genome equivalents (i.e. a 95% probability of having any specific genomic fragment present). The DC3000 clones, in E. coli, are currently arrayed in fifty 384-well microtiter plates and will be replicated into new plates for overnight growth in LB medium. Genomic inserts will be amplified directly from the overnight culture using vector-targeted primers and the resulting PCR products will be purified on Sephadex columns using a TECAN robotics unit available at BTI. With a GMS microarrayer, approximately 4 nl of each PCR product will be spotted onto silanated glass slides at a pitch of 200 microns. At this spacing, it is possible to place 10,000 spots in a 2 x 2 cm square. For preparation of high quality bacterial probes it is necessary to have very pure and undegraded mRNA. To isolate the RNA we will use the method involving hot phenol and agitation with glass beads that has proven successful for previous bacterial microarrays analysis (162, 186). Labeled cDNA will be prepared from DC3000 total RNA by a first-strand reverse transcription reaction using random primers and the fluorescent nucleotide analogs Cy3 and Cy5 (186). After hybridization and washing, the slides will be analyzed using a ScanArray 5000 confocal laser scanner; signal intensity and background measurements will be examined using the program Scanalyze (http://rana.stanford.edu). There are several reports describing methods to analyze bacterial microarrays and we will rely on these techniques and keep abreast of new software that becomes available (7, 38, 186). DC3000 clones that hybridize differentially to the wild-type probes will be sequenced and compared against microbial databases and with the genome sequence of DC3000 as it becomes available. 2d. Perform initial analysis of all candidate effector genes in preparation for subsequent objectives. Similarity searches using NCBI’s BLASTP, BLASTX and PHI-BLAST will be performed to identify any proteins in the databases that have similarity to candidate effector genes (195). In addition, BLAST searches will be carried out against the numerous unfinished microbial sequencing projects currently in progress. ORF-specific primers will then be used to subclone the genes into pFLAG-CTC resulting in a C-terminal FLAG epitope fusion (69). We will then test the ability of the FLAG-tagged protein to be secreted from E. coli(pCPP2156), which expresses the E. chrysanthemi Hrp system (69). 2e. Perform more detailed analysis of selected effector genes. We will further explore the structural and localization basis for the virulence activity of selected bacterial effector proteins. The AvrPto protein will be used as a model to develop methodologies that can be applied to other effectors identified from DC3000. For example, we are using a random mutagenesis approach to identify the sequences required for the virulence activity of AvrPto. We previously generated an avrPto mutant library that was used successfully for the identification of motifs required for the avirulence activities. This mutant library will be screened for mutants that have lost the virulence activity in tomato plants lacking Pto but still carry the avirulence activity in tomato plants containing Pto. The rationale for structure-function 12 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI studies defining virulence-specific motifs of AvrPto and a limited set of other effector proteins is that such motifs may facilitate further genomic analysis of effector genes in phytopathogenic bacteria. Additional effector proteins will be chosen for this analysis primarily on the basis of their contribution to virulence. Proper subcellular localization in the host is likely to be another important aspect of effector activity. The conserved myristylation motif (Met-Gly-x-x-x-Ser/Thr/Val-) has been identified in several bacterial Avr proteins. This motif is essential for both the virulence and the avirulence functions of AvrPto, as described above. We will determine how many other DC3000 effector proteins contain the myristylation motif. The functional relevance of this motif will be examined by replacing the Gly residues with Ala using sitedirected mutagenesis. The resulting mutant will be tested for virulence and avirulence by using assays involving P. s. tomato strains and Agrobacterium-mediated transient expression. We are also developing additional methods for localization of Avr proteins in the plant cell involving GFP. Successful development of these methods will greatly simplify the procedures for effector protein localization and myristylation studies and similar analyses will be applied to any effector proteins that carry nuclear localization signals or other eucaryote targeting signals. A better knowledge of the relationship between such motifs and biological activity will also aid genomic analysis of effector proteins in phytopathogenic bacteria. 2f. Identify additional candidate virulence factors by analysis of the DC3000 genome. The output from the primary annotation of the DC3000 genome described above will be analyzed for ORFs with similarity to known or potential virulence-related sequences in plant and animal pathogens, including those directing toxin synthesis (in addition to coronatine) (14), phytohormone synthesis (34), resistance to antimicrobial peptides (113), resistance to reactive oxygen and nitrogen intermediates (76), protein secretion systems in addition to the Hrp system (19, 137, 187), plant cell wall-degrading enzymes (11), and type III effectors (89). The rapid progress in genomic analyses of animal pathogens will further increase the power of this approach, but we will be equally interested in virulence factors that are completely novel. We will search for these on the basis of their differential presence in: (i) DC3000 but not P. aeruginosa or P. putida, (ii) the two plasmids in DC3000 (because many avr genes are known to be plasmid-borne), (iii) pathogenicity islands or islets (which can be identified by several criteria and may be marked by inclusion of a known virulence factor gene)(68, 94), (iv) or in association with virulence-related mobile genetic elements that are found to be commonly linked to virulence factor genes. 2g. Identify additional candidate virulence factors by microarray analysis of genes specifically expressed at 18oC or in a manner dependent on GacA or other global regulators of virulence. Because of the importance of cool temperatures for disease development and the corresponding thermoregulation of coronatine production and Hrp secretion functions in DC3000 (141, 169), we will use microarray analysis to identify genes that are expressed at 18oC but not 28oC during growth in apoplastmimicking media (91). We will similarly analyze wild-type and gacA mutant strains of DC3000 because of the possibility that the gacA-dependent regulon in DC3000 has a larger role in symptom production than in parasitic growth. The relative importance in virulence of GacA appears to vary among P. syringae pathovars (185), and it will be tested for DC3000 as part of this objective. If gacA mutation has no effect on virulence, we will test other potential virulence regulators, which will be sought in the next objective. We are encouraged in this search by the seminal finding that the regulons controlling Hrp-mediated parasitic growth and lesion formation are distinct in at least some strains of P. syringae and by the conceptual importance of identifying all of the members of these regulons (184, 185). 2h. Identify additional candidate regulators of virulence by analysis of the DC3000 genome (and microarray analysis of regulons expressed in planta). The annotated DC3000 genome also will be analyzed for genes with potential regulatory roles relevant to virulence, including algZ, gacA-gacSsalA (discussed in the previous objective), rpoN, rpoS, sigE (algT), recA, dam, rsmA, rsmB, and ahlI (for the synthesis of a novel acyl homoserine lactone)(79, 104, 185). In addition, in a subsequent objective we will identify genes expressed in planta by microarray analysis. It is likely that this analysis will yield regulons in addition to those involving low-temperature growth, HrpL, and GacA. For example, there may be major shifts in bacterial metabolism reflecting bacterial nutrition in planta in comparison with growth in medium mimicking apoplastic fluids (162). Regulators predicted to control these metabolic shifts may then be sought in the genomic sequence. 13 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 2i. Construct key DC3000 mutants. The search for bacterial genes involved in plant interactions described in the previous objectives will likely yield a plethora of candidates. Our strategy for efficient functional genomics analysis of these candidates will initially involve constructing polar mutations with omega fragments (135), which we will use to disrupt entire polycistronic operons, or to construct large deletions affecting, for example, multiple candidate effector genes in a putative pathogenicity islet. We have used the later approach successfully to demonstrate that the genes in the DC3000 Hrp pathogenicity island conserved effector locus make a collectively significant contribution to virulence (4). These initial mutants will then be analyzed carefully for any changes in their interaction with plants using the molecular and cell biological assays described in objective 3. Any mutants showing a plant interaction phenotype will be subjected to more detailed genetic analysis through the construction of nonpolar mutations with tools such as the aphA-3 cassette (121). 3. Characterize the interactions of tomato and DC3000 by microarray analysis, identify novel attack and defense regulons in both microbe and plant, develop novel bioinformatics-compatible cell biological assays and functional genomics tests. 3a. Gene expression profiling of tomato responses to diagnostic DC3000 strains. As part of an ongoing NSF-funded tomato genomics grant, the Martin laboratory is developing microarrays with 8,000 tomato cDNA inserts (see Preliminary Data; we will obtain these arrays from the BTI Center for Gene Expression Profiling at a cost of $300 per slide). A large amount of pathogen-responsive gene expression data will be available for this 8,000-gene set over the coming year and these benchmark data will be useful for comparisons with specific effector proteins and defined DC3000 mutants. To examine plant response to DC3000 strains, bacteria will be vacuum-infiltrated into 5-week-old tomato plants and RNA will be isolated from the inoculated leaves at several time points (e.g. 0, 2, 4, 12, 24, and 48 hours). PolyA+ RNA will be prepared from the mixed plant/bacterial leaf material and labeled by reverse transcription with poly dT(16) oligomers and fluorescent nucleotides Cy3 or Cy5. Hybridization, washing and analysis will be essentially the same as for the DC3000 microarrays described above. Our initial experiments will involve side-by-side inoculations of tomato leaves with the DC3000 wild-type strain and the DC3000 hrp/hrc mutant. This will provide baseline data for the differences in plant gene expression that are attributable to all bacterial proteins secreted by the Hrp system (i.e. both effector proteins and proteins that constitute the Hrp secretion apparatus). Similar analyses will be done with a DC3000 GacA mutant or similar regulatory mutant that is differentially reduced in symptom production rather than parasitic growth. In each case we will use either the Scanalyze (http://rana.stanford.edu) or GeneCluster programs (http://www.genome.wi.mit.edu) to define sets of coordinately-regulated genes (the "regulons"). We cannot predict the number of these regulons but our efforts will focus on 4-6 that show the most interesting regulation (e.g. very rapid induction, biphasic induction, suppressed by pathogen, etc.). Identification of these regulons will eventually lead to the characterization of the promoter cis elements that are involved in their coordinated regulation (see below). 3b. Develop second generation microarrays with subset of tomato genes that are expressed in response to diagnostic DC3000 strains. The microarray analyses performed above will yield perhaps 200 tomato genes that are differentially expressed during the full range of major possible interactions with DC3000. cDNAs corresponding to these genes will be rearrayed to produce a second generation microarray, which then can be used more economically in subsequent experiments. 3c. Perform microarray analysis of DC3000 genes expressed during association with tomato cells. As an important prelude to this objective, we will construct a DC3000 strain expressing a plasmidborne PhrpA::gfp fusion employing a gfp mutant that produces an unstable GFP (kindly provided to us by Steve Lindow). This will enable us to determine whether hrp genes are expressed maximally only in those bacteria that are (and remain) in contact with plant cells and whether hrp gene expression is transient in planta. We will perform a series of pilot tests using this PhrpA::gfp reporter in conjunction with an appropriate color-variant GFP that is constitutively expressed (see subsequent objective) to determine the optimum procedure for recovering bacteria from infected leaves, for example by centrifugal flushing of apoplastic fluids (39). As an alternative, we will use the PhrpA::gfp reporter to examine hrp expression in response to cultured tomato cells, and these may serve as the source of plant-induced bacteria. Assuming that infected leaf tissue can be used, we will sample bacteria at 0, 2, 4, 12, 24, and 48 hours 14 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI after inoculation (the same intervals used in the tomato microarrays described above) and submit them to microarray analysis as described. We will test both DC3000 (HR-eliciting) and DC3000 avrPto/avrPto2 (pathogenic). 3d. Develop second generation DC3000 microarrays with subset of disease-related ORFspecific fragments. The earlier microarray experiments that profile HrpL and GacA-dependent DC3000 gene expression in culture and the DC3000 gene expression in planta are expected to yield a set of 150200 bacterial genes that are differentially regulated. When the DC3000 genome sequence is available, we will develop ORF-specific PCR primers to produce microarrays of the entire ORF of each of these genes. These smaller arrays will be used for additional experiments to follow up (and confirm) our initial gene expression profiles, and they will be used to examine bacterial gene expression changes that result from mutations in putative regulatory genes and in response to tomato plants that have altered defenses, as described below. 3e. Develop GFP-labeled "reporter bacteria." We have already shown that constitutively expressed S65T GFP can be used to observe DC3000 colonies in undisturbed plant leaves by laser-scanning confocal fluorescence microscopy (59). The assays proposed in subsequent objectives will involve host cells expressing GFP, and to prepare for that work we will test GFP color variants such as YFP and CFP in DC3000 (52, 75). These will help us to differentiate fluorescent signals from microbe and plant, and they will enable us to examine the spatial interactions of different DC3000 mutants in host leaves. For example, P. s. syringae hrp and gacA mutants do not produce lesions in bean leaves, but mixtures of these mutants regain this ability (80). In exploring pathogenesis at the cellular level with DC3000, we feel that it would be informative to observe the fate of similar mutant mixtures as well as individual mutants in relation to plant cells in living plant tissue. 3f. Construct transgenic tomato "cell biological indicator plants" with GFP marking diagnostic subcellular structures and responses. The rapidity of the earliest host responses in an incompatible interaction suggests that cellular remodeling of pre-existing components must take place. It is also uncertain as to which events are cell autonomous, and what cell-cell communication occurs to initiate secondary responses in cells removed from the site of bacterial attachment. We hypothesize that there is a spectrum of subtle, but perceptible, cell responses when faced with a compatible, incompatible (HR-eliciting) or nonpathogenic interaction. To begin to test this hypothesis, six indicator plant lines will be generated to express: (i) Pti4-YFP and Pin2-CFP: transcriptional fusions of the tomato Pti4 promoter (salicylic acid (SA)and ethylene-induced) (197) to yellow-shifted GFP (mYFP, engineered for high expression in plants) (75) and the tomato Pin2 promoter (jasmonic acid (JA) induced) (33, 133) to cyan-shifted GFP (mCFP, engineered for high expression in plants) (75); (ii) GFP-NLS: sGFP (engineered for high expression in plants) (30) targeted to the nucleus by fusion to the nuclear localization signals in squash leaf curl virus NSP (148); (iii) GFP-mTn: sGFP fused to the F-actin binding domain of mouse talin which has been used to visualize the actin cytoskeleton in a variety of cells, including plants (105); (iv) GFP-MBD: sGFP fused to the microtubule binding domain of mammalian MAP4, which has been used to visualize microtubules in plants (117); (v) GFP-aERD2: sGFP fused to aERD2, the Arabidopsis H/KDEL receptor homologue (targets to ER and Golgi) (20, 149) ; and (vi) hsr203-GFP: transcriptional fusion of the tomato hsr203 promoter (induced during PCD in incompatible interactions) (134) to sGFP. Seedlings from each Indicator line will first be tested by inoculation with DC3000 (positive control for defense responses), DC3000 avrPto/avrPto2, and DC3000 hrp/hrc to define the range of cellular responses and determine which may be specific or common for compatible, incompatible or nonpathogenic interactions. A lower leaf on young seedlings (4 leaf stage) will be inoculated by spraying with the appropriate DC3000 strain in Silwet. Inoculated leaves will be removed at 3 and 6 hr postinoculation, and imaged to detect any changes when compared to mockinoculated leaves. Once the response range has been determined, we will examine systemic leaves for any relevant alterations at different times postinoculation and leaves inoculated by localized infiltration for alterations in cells distal to those in contact with bacteria (secondary cell-cell signaling effects). All expression constructs use forms of GFP that are engineered for high level expression in plants and have been successfully used in epifluorescence or confocal imaging in intact plants. Leaves will be mounted in water and examined using a long-working-distance water immersion objective to allow for deep focusing into an aqueous sample (e.g. Nikon 60-X planapochromat, aperture 1.2). This should allow imaging to a depth of ~80-100 m into the tissue (64, 75), which is sufficient to image down to mesophyll cells (the site of bacterial 15 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI infection). Leaves from the GFP-NLS and hsr203 lines will be imaged using an epifluorescence microscope. In general, subcellular changes in the other Indicator lines will be detected by confocal microscopy. These 6 indicator plant lines are designed to detect a spectrum of potential cell responses from gene induction associated with systemic acquired resistance or PCD and nuclear changes, to endomembrane and cytoskeletal alterations. The dual Pti4-YFP and Pin2-CFP plants, and hsr203-GFP plants will look at the induction of specific signaling pathways, but the other 4 lines will examine cellular remodeling in response to pathogen perception. These studies will establish the feasibility of this approach and provide new basic information on plant-pathogen interactions at the cellular level. Induction of Pti4-YFP and Pin2-CFP expression will be detected by confocal imaging using appropriate filters and Kr/Ar lasers for sequential excitation, and the appropriate dichroic beamsplitter and emission filters (52, 75). This pair was selected because their spectral differences allow for clean discrimination of signals in dual imaging. The potential to express both in the same cell will allow direct comparison of the induction of SA (Pti4-YFP expression, and potentially systemic acquired resistance and JA (wound response) (Pin2-CFP expression). Nuclear migration has been reported in fungal-host interactions (71), but it remains unclear whether this is a more general plant response to microbes or pathogens. GFP-NLS is designed to detect nuclear migration away from or towards bacterial attachment site, and would also reveal gross nuclear alterations (e.g. swelling or distention). To construct GFP-NLS, we will fuse the N-terminal two-thirds of NSP to sGFP so that the fusion protein is ~48kD, large enough not to passively diffuse between the cytoplasm and nucleus (64). GFP-mTn and GFPMBD are designed to decorate actin microfilaments and tubulin-containing microtubules, respectively. Since each contains only the relevant binding domain neither perturbs cytoskeletal structure or dynamics (105, 117). These Indicator lines will assess changes in orientation, assembly and polarization of the microfilament and microtubule networks in response to DC3000 strains, and in particular localized changes at the sites of bacterial attachment. GFP-aERD2 is targeted to both the ER and Golgi in plants (20, 149), and thus will detect changes in ER networks and Golgi structure, as well as the generation of vesicles associated with directed transport to the site of bacterial attachment and directed secretion. Changes in the cortical ER would be of particular interest as this subcellular compartment is important in cell-to-cell movement protein function for a diverse set of plant viruses (108). PCD is an important aspect of the defense response in incompatible interactions, and can be uncoupled from defense gene activation during HR (40, 134). hsr203 induction strongly correlates with PCD in tobacco and tomato (134), thus the hsr203-GFP line will specifically look at induction of PCD. An alternative approach is to express a CFP-YVAD-YFP fusion in tomato and assay for cleavage of the caspase sensitive YVAD linker by FRET analysis (YFP fluorescence uncoupled from CFP excitation) (116). However, given inconsistencies in inhibitor studies that examined caspases in PCD during HR (40), it is not certain that YVAD or DVED proteolytic activity is present. Should additional evidence demonstrate either or both of these activities exist during HR, we will consider this approach. Once we have determined the range of alterations for each line, we will develop a quantitative scale to express the data in a bioinformatics-compatible manner. For example, expression of Pti4-YFP will be scored on a scale from “–“ to “++++” for no to maximal expression, with intermediate levels being scored as +, ++ or +++. Similarly, nuclear migration or reorientation of actin filaments will be scored from “–“ for no change relative to controls to “++++” for maximal migration away from or reorienting towards the bacterial attachment site, and intermediate changes scored accordingly. 3g. Perform microarray analysis of tomato responses to DC3000 mutants and isolated effectors. The interaction-specific second generation tomato microarrays previously described will be used to profile plant gene expression in response to DC3000 strains that have mutations in specific effector or virulence regulatory genes. Also, for a limited number of effector proteins we will examine host responses when the effectors are expressed directly in plant cells in the absence of DC3000. Methods available for this expression are either PEG transformation of protoplasts (2) or DEX promoter-inducible expression in transgenic plants (120). Alternatively, we may use Agrobacterium transient expression (161) or P. fluorescens(pCPP2071), which carries the functional P. s. syringae 61 hrp/hrc cluster without an effector gene and can be used to deliver heterologous effectors (6, 63). These approaches will be assessed for convenience and efficacy with regard to the timing of induction and background defense induction. In summary, over the five year project period we will define the coordinately-regulated set of plant genes that respond to DC3000 and several mutant derivatives and individual effector proteins. These experiments will lead to the discovery of new plant genes that are potentially involved in defense against bacterial pathogens and, in addition, they will be used to delineate key cis-elements of a representative set of these genes. 16 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 3h. Identify cis-elements and transcription factors controlling tomato regulons that respond to DC3000 attack. The tomato microarray experiments described above will yield several regulons of particular interest. Genes within a regulon are likely to be controlled by common regulatory pathways, and are thus expected to possess similar critical promoter elements. Our goal is to identify these so they may be used to identify the factors which regulate expression of genes within each regulon. We aim to analyze 3 to 4 distinct regulons that appear particularly relevant to the interaction, each composed of several or more tomato genes. Where possible, it will be important to confirm that the altered mRNA levels observed after elicitation result from changes in transcription, rather than other factors that affect RNA accumulation levels. Therefore, genes within each regulon will be tested in run-on transcription assays using nuclei obtained from treated versus control leaves (175). One or two representative genes will be selected from each regulon based on their robust behavior in microarray and run-on transcription assays. These will be used to obtain the flanking genomic sequences including promoters, by screening tomato genomic libraries with cDNA probes, or with 5’ RACE (rapid amplification of cDNA ends) (150). At least two approaches will be used to identify critical cis-acting promoter elements involved in regulon-specific gene induction. First, regulon-specific tomato genes will be used to query the Arabidopsis genomic sequence database, with the goal of identifying orthologous Arabidopsis genes and their promoters. The set of Arabidopsis promoters will then be analyzed to identify shared sequence signatures (26, 44, 171) that link the orthologs into sets corresponding to specific tomato regulons. These elements will also be looked for in the tomato promoters. Conserved sequence elements (outside of those found in most promoters) that define regulon-specific sets will be tested for relevance in tomato by construction of promoter-reporter constructs that contain the native tomato promoter fused to easily assayed reporter genes, such as luciferase or uidA encoding -glucuronidase (GUS). The constructs will be transiently introduced into tomato protoplasts using PEG transformation, or into tobacco using potato virus X. Transgene-containing tomato cells will be exposed to DC3000, its mutant derivatives or more refined agents to assess induction of the reporter. Putative critical cis-elements will be knocked out by sitedirected mutation of the promoter to determine whether such disruptions affect the test promoter. An alternative method of promoter analysis will be employed, should studies of orthologous genes fail to yield useful information. Tomato promoters will be fused to easily assayed reporter genes, and transiently introduced into plant cells as described. Treatments with various elicitors will be assessed for their effect on reporter gene expression and optimized to mimic expression of the transgene with native genes in a regulon of interest. 5’ and 3’ promoter deletion analyses followed by quantitative reporter gene expression analysis will be performed to quickly delimit promoter regions important for a given induction. Regions shown to play a role in regulon-specific gene expression will be more closely analyzed by in vitro mutagenesis of that promoter region, followed by transient transformation and quantitation of reporter expression. All quantitative transient assays will be performed using an internal standard construct cotransformed into plant cells, to permit compensation for variability in transformation efficiency or other factors. For example, co-transformation of a test construct (e.g. with a luciferase reporter) with a standard amount of a constitutive positive-control construct (e.g. CaMV 35S promoter-uidA) allows normalizing expression of the test construct to GUS expression, which can be accurately measured using any of a number of other chemiluminescent or fluorogenic substrates (such as 4-methylumbelliferyl-b-Dglucuronide (MUG). 3i. Identify transcription factors controlling tomato regulons that respond to DC3000 attack. The cis-elements defined above will be used to identify cognate transcription factors. A number of biochemical or genetic methods have been used to identify proteins that interact with specific DNA sequences. We will employ the yeast one-hybrid system as our initial system aimed at discovering regulon-specific transcription factors (101). One such system employs a GAL4-activation domain (AD)fusion plasmid into which would be cloned a tomato cDNA library (e.g. Clontech Matchmaker system). The cDNA-AD fusion library is transformed into a yeast strain containing a tomato promoter element of interest (at least 3 tandem copies) upstream of a weak promoter driving HIS3 and lacZ reporter genes. Interaction of a cDNA (fused to AD) with the promoter element confers histidine prototrophy on the recipient yeast strain. Such proteins are candidate regulatory factors controlling regulon-specific gene expression. 17 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 3j. Construct transgenic tomato plants altered in their production of key regulators of defense and susceptibility. Candidate regulatory factors will be validated by gel retardation assays to detect interaction of a putative regulatory factor with the promoter element. More definitive evidence for a role of the transcription factor will be through demonstration that regulation of genes within a relevant regulon can be perturbed by constitutive expression of the factor or by disruption of its production through viral-delivered antisense or sense (VIGS) (12) approaches, as described above. Based on these experiments, a limited set of transgenic plants will be constructed, in which production of the factor is altered through constitutive expression or antisense disruption. 3k. Test the role of selected regulons and interaction factors in both plant and bacterium with bioinformatics-compatible cell biological assays and microarray analyses. The previous objectives have described the development of a set of tools (second generation microarrays and cell biological assays) that will be used at this point in the project to systematically develop and test hypotheses regarding the factors underlying bacterial pathogenesis, plant susceptibility, and plant defense. The tests will involve bacteria and plants genetically altered in their production of candidate interaction factors, as described above, and the results will be entered in the community database described in the next objective. A key aspect of this objective is the development of a working model of the interaction at the molecular/cellular level. For example, we may find that the reason that AvrPto has no (currently detectable) virulence phenotype in DC3000 (in contrast to strain T1) is that DC3000 happens to possess a functionally redundant effector. By finding molecular/cellular phenotypes for effectors like AvrPto, we will begin to relate effectors to specific stages in pathogenesis and to groups of effectors that may redundantly control that stage, just as multiple Yersinia Yops function in different ways to disrupt the cytoskeleton in macrophages (89). 4. Develop a community-oriented Pseudomonas-Plant Genome Interaction Database to support the functional genomics of interorganismal interactions and an educational outreach initiative involving development of a new high school level integrated science/math/social studies course on “Genomics: Applications and Implications.” 4a. Develop a community-oriented Pseudomonas-Plant Genome Interaction Database to support the functional genomics of interorganismal interactions. The genomic sequence of P. syringae will be distributed in annotated form from TIGR’s gene index and as raw data from GenBank. However, to integrate the functional genomics data from the project and maximize its availability, we propose to establish the Pseudomonas-Plant Genome Interaction Database (PPGID), a central, curated electronic resource that will focus on the genomics of pathogen-host interactions. The PPGID will serve as a growing, organized, community center and a knowledge clearinghouse. The implementation plan for PPGID will be modeled on the P. aeruginosa database at http://www.pseudomonas.com, which has recruited volunteers from the community to provide annotation and other coordination activities around their database site. Community involvement serves to increase awareness and support, improve reliability and quality, provide critical feedback for design decisions, and offset some of the costs of curation and development. The PPGID will have the following objectives: (i) Create a web-based curator-moderated community annotation system for new data; store and distribute results of gene expression profiling experiments and functional analyses; and link information on gene expression and molecular targets in the host with cognate data on gene expression and effectors in the pathogen. (ii) Provide a web-based forum for discussion of hypotheses regarding emerging data, standardization of informatics tools and laboratory methods, and other important community issues. (iii) Provide a practically useful, webaccessible data repository for annotated data generated by this project. (iv) Establish an outreach mechanism to deliver information about plant pathogenesis to extension agents and non-specialists including K-12 educators and students. (v) Create a new database and associated user interfaces for project-related data by leveraging existing infrastructure (see facilities statement), including data and informatics from TIGR, and sequence information from NCBI and “SynTom” database for Solanaceae sequences (vi) Identify useful linkages to external sources of information on sequences, bibliographic references, metabolic pathways, basic biology of model organisms, and general mechanisms of plant pathogenesis. Further information on informatics is given below. We will pursue other opportunities for expanding the informatics component of this project to address the remaining objectives (e.g. NSF REU funding for undergraduate participation, USDA-ARS funding for long-term curation and delivery). 18 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 4b. Develop a new high school level integrated science/math/social studies course on “Genomics: Applications and Implications.” Based on discussions with Drs. Peter Bruns and Rita Calvo who direct the Cornell Howard Hughes Medical Institute (HHMI) Program, and with local high school and middle school science and math teachers, one of whom is active on the NY State Board of Education, we have established excitement about and interest in developing a one semester integrated cross-disciplinary course on genomics and genetically modified organisms. The emphasis will be on plants. The course, to be taught at the junior or senior high school level, will be developed by teams of high school science, math, social studies and English teachers together with Cornell and BTI co-PIs on this grant and the Cornell HHMI Science Education program. Such a course will meet NY State requirements in science education and the current emphasis on more integrated, interdisciplinary teaching that better enables students to ‘make connections.’ Three teams of 3 teachers (science, math, and social studies or English), with support from University faculty, will develop this course, thus ensuring that it is interdisciplinary. The scientific subject fits best with our high school curriculum (students are introduced to genetics in 9th grade, and the relevant math and social studies are taken even later) and has obvious strong links to math (bioinformatics, databases), social studies (social and environmental impact, legal issues) and English (a wealth of media presentations and literature that depict this subject in different ways). Course materials and hands-on activities (labs) will be developed for use in a standard or advanced course, such that individual ‘modules’ can also be adapted for use by high school teachers in their own courses. We will provide (i) background information on the relevant subjects in the form of workshops and written materials for teachers; (ii) teaching materials in support of each of the separate subjects; and (iii) inquiry-based activities for each subject in an integrated plan so that what is done in one subject supports and is relevant to the others. The PPGID developed under this proposal will further support this course by providing additional educational materials and data sharing devices so that high school students at different locations can share data, some of it in real time, and develop further projects together and with faculty. In the first summer of this grant 9 local rural high school teachers (3 each for science, math and social studies/English) and Cornell faculty will meet to further define the scope of the course and identify the elements to be covered. During the following academic year, these teachers will work together in teams of 3, and with Cornell faculty, to develop the course materials, which they will assemble in a 1 week development workshop to be held the following summer on campus. These teachers will test the new course the following academic year, and during the next summer, with the support of Cornell faculty, hold a presentation workshop in which the completed unit and web-based materials are presented to new high school teachers who will be recruited in teams. We will provide ongoing support for the unit and for bringing new teachers into the program, and are exploring additional sources of funding (e.g. the Park Foundation). This program will become an ongoing part of the Cornell HHMI Program. 4c. Continue TIGR educational outreach activities. TIGR has a summer internship program that taps into local high schools and colleges for bright, promising students wanting experience in genomic research. This last summer, Dr. Buell mentored a high school intern to work on the Arabidopsis Genome Project at TIGR. TIGR is currently sponsoring a graduate student intern from Michigan State University DOE-Plant Research Laboratory as part of a doctoral biotechnology internship program. This intern has been working on bioinformatics in the Arabidopsis and tomato genome projects at TIGR, with an emphasis on genes involved in disease resistance. As we have interested students, TIGR is willing to have interns participate in the sequencing and annotation of DC3000. TIGR is participating in the newly established Computational Biology Program at Johns Hopkins University. Steven Salzberg, TIGR's Director of Bioinformatics, is on the executive board of this program, and the program curriculum calls for graduate students and postdocs to spend 6-month rotations at TIGR as part of their training. These rotations will teach the students about HTGS and about bioinformatics. The program is fully funded by a grant from the Burroughs-Wellcome Foundation. TIGR has a Conferences, Education, & Training department that serves two functions. First, it instructs in-house staff on methodologies for highthroughput sequencing. Second, it sponsors workshops for local elementary, middle, and high school teachers to gain experience in molecular biology and genomics. TIGR also sponsors one teacher intern each summer as part of its Summer Fellows program. Faculty at TIGR participate in local science discussions as requested by local colleges and governments. 19 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI INFORMATICS TIGR: We will utilize the same database structure to sequence the ~6 Mb genome of DC3000 that we have utilized for other microbial genomes. Through our 10 completed genomes and our 21 on-going microbial genome projects, we have standard operating protocols, support personnel, and other infrastructures (databases, in-house tracking system, annotation tools) in place that can be readily transferred to the DC3000 project. TIGR's database environment is state-of-the-art, with processing distributed among five servers, each running the latest release of Sybase Adaptive Server Enterprise. Our primary production server resides on a Sun UltraEnterprise 3000 with six CPUs, four gigabytes of memory and over 500 gigabytes of available disk space. All of TIGR's 140+ databases are stored in a dedicated RAID-5 enclosure, with a fiber-channel connection to the server, for the ultimate in performance, safety and availability. The annotation process for microbial genomes begins gene finding using GLIMMER (Salzberg et al. 1998) and predicted open reading frames (ORFs) are searched against a non-redundant amino acid database of all currently known sequences. The search method used is the BLASTP algorithm followed by a modified Smith-Waterman alignment (Waterman, 1988). TIGR's parallel virtual machine cluster for conducting these searches contains 52 x 450/550 MHz Pentium II/III class processors with combine total of 14 gigabytes of random access memory (RAM) and a 250 gigabyte ultra fast shared (NetApp) network storage device for database access and data storage. With this clustered system, approximately 2500 ORFs can be searched per hour. Following a preliminary automated data analysis process that assigns putative functions to the ORFs based on these searches, the data is curated by a team of experts. These automated assignments are confirmed by looking at the original search results as well as other evidence, such as Hidden Markov Models searches and Prosite matches. The data is analyzed and updated through both robust web-based graphical user interfaces and standalone JAVA applications. Small Genome Control, which is a suite of Perl and C scripts managed by a UNIX makefile, is automatically run on the data. Small Genome Control analyzes the data for features such as protein molecular weights and transmembrane domains, identifies any putative sequencing errors in the underlying DNA sequence, and manages changes to any data on a daily basis (please see http://www.tigr.org/~ehickey/SGC/SGC_abstract.html for more details). Pseudomonas-Plant Genome Interaction Database: When annotation of DC3000 is complete, TIGR personnel will collaborate with Sam Cartinhour to incorporate the DC3000 annotation into the PPGID to be developed at Cornell. As part of its Comprehensive Microbial Resource database, TIGR will continue to improve the annotation of DC3000 outside of the grant period. Thus, we envision a continual and close collaboration between TIGR and the PPGID such that both databases benefit from their own expertise in microbial annotation and pathology, respectively. The specific plans for PPGID will be staged as follows. As early as possible, project PIs will identify volunteers from the community to form an oversight committee for PPGID. The committee will be composed of 5 scientists external to the project and will provide guidance and feedback for the goals and content of the PPGID site. Bob Hancock, developer of the P. aeruginosa site at www.pseudomonas.com has agreed to provide guidance in our early stages of planning. A 0.5 FTE PPGID curator will be hired to establish a PPGID WWW site and a listserv for community discussion. The curator will also serve in an outreach capacity to engage researchers interested in participating in annotation, coordinate closely with counterparts at TIGR as sequence data is generated, and investigate linkages to other related web sites. A 0.5 FTE software developer will also be hired to begin development of server-side infrastructure and annotation system in collaboration with the PPGID curator. We will use Oracle version 8 as a development environment (already purchased for another project) and take advantage of our existing server hardware (the USDA-ARS 48-500 MHz processor NT cluster maintained at the Cornell Theory Center with 48 GB RAM and approximately 500 GB disk). Consultation with the project PIs and oversight committee will be important in designing a practical, relevant system for working biologists. In subsequent years, remaining informatics funds will be used to hire talented undergraduate programmers from Cornell and Wells College working under the supervision of the software engineer. In addition, projects will be designed to involve M. Eng. Students to tackle more complicated development tasks. The PPGID team will produce a preliminary implementation of a PPGID database and a basic user interface leveraging existing infrastructure and tools developed by other projects such as HyperSQL (see documentation at http://mgd.nacse.org/hsql/docs/manual/hypersql_language_reference.html/ and example at http://mgd.NACSE.ORG/hyperSQL/lichenland/). The curator will continue site enrichment throughout the lifetime of the project by establishing a repository for data flowing from the laboratory, writing 20 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI documentation, identifying and integrating relevant information, promoting community involvement and (in later years) creating outreach materials. The CBCG team already includes experienced personnel involved in curating and maintaining databases for Rice and small grains (e.g., wheat, barley and oats). The curator and programmers will be sited within the ARS Center for Bioinformatics and Comparative Genomics and the Cornell Institute for Computational Genomics in order to maximize access to existing expertise and resources for informatics. 21 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI References to Project Description 1. Aarts, N., M. Metz, E. Holub, B. J. Staskawicz, M. J. Daniels, and J. E. Parker. 1998. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 95:10306-10311. 2. Abel, S., and A. Theologis. 1994. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J. 5:421-427. 3. Agrios, G. N. 1988. Plant Pathology, Third Ed. Academic Press, San Diego. 4. Alfano, J. R., A. O. Charkowski, W.-L. Deng, J. L. Badel, T. Petnicki, K. van Dijk, and A. Collmer. 2000. The Pseudomonas syringae Hrp pathogenicity island has a tripartite mosaic structure comprised of type III secretion genes bounded by exchangeable effector and conserved effector loci that contribute to parasitic fitness and pathogenicity in plants. submitted 5. Alfano, J. R., and A. Collmer. 1997. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J. Bacteriol. 179:5655-5662. 6. Alfano, J. R., H.-S. Kim, T. P. Delaney, and A. Collmer. 1997. Evidence that the Pseudomonas syringae pv. syringae hrp-linked hrmA gene encodes an Avr-like protein that acts in a hrp-dependent manner within tobacco cells. Mol. Plant-Microbe Interact. 10:580-588. 7. Alland, D., I. Kramnik, T. R. Weisbrod, L. Otsubo, R. Cerny, L. P. Miller, W. R. Jacobs, Jr., and B. R. Bloom. 1998. Identification of differentially expressed mRNA in prokaryotic organisms by customized amplification libraries (DECAL): the effect of isoniazid on gene expression in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U S A 95:13227-32. 8. Anderson, D. M., D. E. Fouts, A. Collmer, and O. Schneewind. 1999. Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc. Natl. Acad. Sci. USA 96:12839-12843. 9. Ausubel, F. M., R. Brent, R. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1994. Current Protocols in Molecular Biology. John Wiley and Sons, New York. 10. Bateman, A., E. Birney, R. Durbin, S. R. Eddy, R. D. Finn, and E. L. Sonnhammer. 1999. Pfam 3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res. 27:260-262. 11. Bauer, D. W., and A. Collmer. 1997. Molecular cloning, characterization, and mutagenesis of a pel gene from Pseudomonas syringae pv. lachrymans encoding a member of the Erwinia chrysanthemi PelADE family of pectate lyases. Mol. Plant-Microbe Interact. 10:369-379. 12. Baulcombe, D. C. 1999. Fast forward genetics based on virus-induced gene silencing. Curr. Opin. Plant Biol. 2:109-113. 13. Bendahmane, A., K. Kanyuka, and D. C. Baulcombe. 1999. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11:781-792. 14. Bender, C. L., F. Alarcon-Chaidez, and D. C. Gross. 1999. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 63:266-292. 15. Bender, C. L., V. Rangaswamy, and J. Loper. 1999. Polyketide production by plant-associated pseudomonads. Annu. Rev. Phytopathol. 37:175-196. 16. Bender, C. L., H. E. Stone, J. J. Sims, and D. A. Cooksey. 1987. Reduced pathogen fitness of Pseudomonas syringae pv. tomato Tn5 mutants defective in coronatine production. Physiol. Mol. Plant Pathol. 30:273-283. 17. Bent, A. 1996. Function meets structure in the study of plant disease resistance genes. Plant Cell 8:1757-1771. 18. Bestwick, C. S., I. R. Brown, M. H. R. Bennett, and J. W. Mansfield. 1997. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9:209-221. 19. Binet, R., S. Letoffe, J. M. Ghigo, P. Delepelaire, and C. Wandersman. 1997. Protein secretion by gram-negative bacterial ABC exporters - a review. Gene 192:7-11. 20. Boevink, P., K. Oparka, S. Santa Cruz, B. Martin, A. Betteridge, and C. Hawes. 1998. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 15:441-447. 21. Bogdanove, A. J., J. F. Kim, Z. Wei, P. Kolchinsky, A. O. Charkowski, A. K. Conlin, A. Collmer, and S. V. Beer. 1998. Homology and functional similarity of an hrp-linked pathogenicity locus, dspEF, of 22 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI Erwinia amylovora and the avirulence locus avrE of Pseudomonas syringae pathovar tomato. Proc. Natl. Acad. Sci. USA 95:1325-1330. 22. Bonas, U., and G. Van den Ackerveken. 1997. Recognition of bacterial avirulence proteins occurs inside the plant cell: a general phenomenon in resistance to bacterial diseases? Plant J. 12:1-7. 23. Boyes, D. C., J. Nam, and J. L. Dangl. 1998. The Arabidopsis thaliana RPM1 disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response. Proc. Natl. Acad. Sci. U S A 95:15849-15854. 24. Brommonschenkel, S. H., and S. D. Tanksley. 1997. Map-based cloning of the tomato genomic region that spans the Sw-5 tospovirus resistance gene in tomato. Mol. Gen. Genet. 256:121-126. 25. Brown, I., J. Mansfield, and U. Bonas. 1995. hrp genes in Xanthomonas campestris pv. vesicatoria determine ability to suppress papilla deposition in pepper mesophyll cells. Mol. Plant-Microbe Interact. 8:825-836. 26. Brzma, A., I. Jonassen, J. Vilo, and E. Ukkonen. 1998. Predicting gene regulatory elements in silico on a genomic scale. Genome Res. 8:1202-1215. 27. Bult, C. J., O. White, G. J. Olsen, L. Zhou, R. D. Fleischmann, G. G. Sutton, J. A. Blake, L. M. FitzGerald, R. A. Clayton, J. D. Gocayne, A. R. Kerlavage, B. A. Dougherty, J. F. Tomb, M. D. Adams, C. I. Reich, R. Overbeek, E. F. Kirkness, K. G. Weinstock, J. M. Merrick, A. Glodek, J. L. Scott, N. S. M. Geoghagen, and J. C. Venter. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii [see comments]. Science 273:1058-1073. 28. Charnock, C. 1998. Characterization of the phytopathogen Pseudomonas syringae pathovar ribicola NCPPB 963. Microbios 94:23-34. 29. Chatterjee, A. K., C. K. Dumenyo, Y. Liu, and A. Chatterjee. 2000. Erwinia: Genetics of pathogenicity factors. Encyclopedia of Microbiology, Vol. 2 (in press): 30. Chiu, W.-L., Y. Niwa, W. Zeng, T. Hirano, H. Kobayashi, and J. Sheen. 1995. Engineered GFP as a vital reporter in plants. Curr. Biol. 6:325-330. 31. Claros, M. G., and G. von Heijne. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. 32. Collmer, A., J. R. Alfano, D. M. Anderson, J. L. Badel, W.-L. Deng, D. E. Fouts, A. H. Rehm, C. M. Rojas, O. Schneewind, and K. van Dijk. 2000. Hrp (type III) protein secretion and the virulence of Pseudomonas syringae and Erwinia chrysanthemi, p. 65-70. In P. J. G. M. de Wit, T. Bisseling, and W. Stiekema (ed.), Biology of Plant-Microbe Interactions, vol. 2. International Society for Molecular Plant-Microbe Interactions, St. Paul. 33. Conconi, A., and C. A. Ryan. 1993. DNase I and micrococcal nuclease analysis of the tomato proteinase inhibitor I gene in chromatin. J. Biol. Chem. 268:430-435. 34. Costacurta, A., and J. Vanderleyden. 1995. Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 2:1-18. 35. Cuppels, D. A., and T. Ainsworth. 1995. Molecular and physiological charaterization of Pseudomonas syringae pv. tomato and Pseudomonas syringae pv. maculicola strains that produce the phytotoxin coronatine. Appl. Environ. Microbiol. 61:3530-3536. 36. De Ita, M., R. Marsch-Moreno, P. Guzman, and A. Alvarez-Morales. 1998. Physical map of the chromosome of the phytopathogenic bacterium Pseudomonas syringae pv. phaseolicola. Microbiology 144:493-501. 37. de Lorenzo, V., L. Eltis, B. Kessler, and K. N. Timmis. 1993. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 123:17-24. 38. de Saizieu, A., U. Certa, J. Warrington, C. Gray, W. Keck, and J. Mous. 1998. Bacterial transcript imaging by hybridization of total RNA to oligonucleotide arrays. Nat. Biotechnol. 16:45-48. 39. De Wit, P. J. G. M., and G. Spikman. 1982. Evidence for the occurrence of race and cultivar-specific elicitors of necrosis in intercellular fluids of compatible interactions of Cladosporium fulvum and tomato. Physiol. Plant Pathol. 21:1-11. 40. del Pozo, O., and E. Lam. 1998. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr. Biol. 8:1129-1132. 41. Delcher, A. L., S. Kasif, R. D. Fleischmann, J. Peterson, O. White, and S. L. Salzberg. 1999. Alignment of whole genomes. Nucleic Acids Res. 27:2369-2376. 42. Delledonne, M., Y. Xia, R. A. Dixon, and C. Lamb. 1998. Nitric oxide functions as a signal in plant disease resistance. Nature 394:585-588. 23 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 43. Deng, W.-L., G. Preston, A. Collmer, C.-J. Chang, and H.-C. Huang. 1998. Characterization of the hrpC and hrpRS operons of Pseudomonas syringae pathovars syringae, tomato, and glycinea and analysis of the ability of hrpF, hrpG, hrcC, hrpT, and hrpV mutants to elicit the hypersensitive response and disease in plants. J. Bacteriol. 180:4523-4531. 44. DeRisi, J. L., V. R. Iyer, and P. O. Brown. 1997. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 278:680-686. 45. Dixon, M. S., K. Hatzixanthis, D. A. Jones, K. Harrison, and J. D. Jones. 1998. The tomato Cf-5 disease resistance gene and six homologs show pronounced allelic variation in leucine-rich repeat copy number. Plant Cell 10:1915-1925. 46. Dixon, M. S., D. A. Jones, J. S. Keddie, C. M. Thomas, K. Harrison, and J. D. G. Jones. 1996. The tomato Cf-2 disease resistance locus comprises two functional genes encoding leucine-rich repeat proteins. Cell 84:451-459. 47. Dow, J. M., and M. J. Daniels. 1994. Pathogenicity determinants and global regulation of pathogenicity of Xanthomonas campestris pv. campestris, p. 29-41. In J. L. Dangl (ed.), Current Topics in Microbiology and Immunology, Vol. 192: Bacterial Pathogenesis of Plants and Animals Molecular and Cellular Mechanisms. Springer-Verlag, Berlin. 48. Duan, Y. P., A. Castaneda, G. Zhao, G. Erdos, and D. W. Gabriel. 1999. Expression of a single, hostspecific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant-Microbe Interact. 12:556-560. 49. Dumenyo, C. K., A. Mukherjee, W. Chun, and A. K. Chatterjee. 1998. Genetic and physiological evidence for the production of N-acyl homoserine lactones by Pseudomonas syringae pv. syringae and other fluorescent plant pathogenic Pseudomonas species. Eur. J. Plant Pathol. 104:569-582. 50. Durner, J., D. Wendehenne, and D. F. Klessig. 1998. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95:10328-10333. 51. Eddy, S. R., and R. Durbin. 1994. RNA sequence analysis using covariance models. Nucleic Acids Res 22:2079-2088. 52. Ellenberg, J., J. Lippincott-Schwartz, and J. F. Presley. 1999. Dual-colour imaging with GFP variants. Trends Cell Biol. 9:52-56. 53. Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, and et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. 54. Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, and et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. 55. Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, and et al. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. 56. Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, J. C. Venter, and et al. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. 57. Frederick, R. D., R. L. Thilmony, G. Sessa, and G. B. Martin. 1998. Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol. Cell 2:241-245. 58. Gage, D. J., T. Bobo, and S. R. Long. 1996. Use of green fluorescent protein to visualize early events of symbiosis between Rhizobium meliloti and alfalfa (Medicago sativa). J. Bacteriol. 178:7159-7166. 59. Galán, J. E., and A. Collmer. 1999. Type III secretion machines: ingenious bacterial devices for protein delivery into host cells. Science 284:1322-1328. 60. Gardner, M. J., H. Tettelin, D. J. Carucci, L. M. Cummings, L. Aravind, E. V. Koonin, S. Shallom, T. Mason, K. Yu, C. Fujii, J. Pederson, K. Shen, J. Jing, C. Aston, Z. Lai, D. C. Schwartz, M. Pertea, S. Salzberg, L. Zhou, G. G. Sutton, R. Clayton, O. White, H. O. Smith, C. M. Fraser, S. L. Hoffman, and et al. 1998. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science 282:1126-1132. 24 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 61. Gatz, C., C. Frohberg, and R. Wendenburg. 1992. Stringent repression and homogeneous derepression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J. 2:397-404. 62. Glickmann, E., L. Gardan, and Y. Dessaux. 1998. Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol. Plant-Microbe Interact. 11:156-162. 63. Gopalan, S., D. W. Bauer, J. R. Alfano, A. O. Loniello, S. Y. He, and A. Collmer. 1996. Expression of the Pseudomonas syringae avirulence protein AvrB in plant cells alleviates its dependence on the hypersensitive response and pathogenicity (Hrp) secretion system in eliciting genotype-specific hypersensitive cell death. Plant Cell 8:1095-1105. 64. Grebenok, R. J., E. Pierson, G. M. Lambert, F. C. Gong, C. L. Afonso, R. Haldeman-Cahill, J. C. Carrington, and D. W. Galbraith. 1997. Green-fluorescent protein fusions for efficient characterization of nuclear targeting. Plant J. 11:573-86. 65. Grimm, C., W. Aufsatz, and N. J. Panopoulos. 1995. The hrpRS locus of Pseudomonas syringae pv. phaseolicola constitutes a complex regulatory unit. Mol. Microbiol. 15:155-165. 66. Grimm, C., and N. J. Panopoulos. 1989. The predicted protein product of a pathogenicity locus from Pseudomonas syringae pv. phaseolicola is homologous to a highly conserved domain of several procaryotic regulatory proteins. J. Bacteriol. 171:5031-5038. 67. Groisman, E. A., and H. Ochman. 1996. Pathogenicity islands: bacterial evolution in quantum leaps. Cell 87:791-794. 68. Hacker, J., G. Blum-Oehler, I. Muhldorfer, and H. Tschape. 1997. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 23:1089-1097. 69. Ham, J. H., D. W. Bauer, D. E. Fouts, and A. Collmer. 1998. A cloned Erwinia chrysanthemi Hrp (type III protein secretion) system functions in Escherichia coli to deliver Pseudomonas syringae Avr signals to plant cells and to secrete Avr proteins in culture. Proc. Natl. Acad. Sci. USA 95:1020610211. 70. Hamilton, A. J., R. G. Fray, and D. Grierson. 1995. Sense and antisense inactivation of fruit ripening genes in tomato. Curr. Top. Microbiol. Immunol. 197:77-89. 71. Hammond-Kosack, K. E., and J. D. G. Jones. 1996. Resistance gene-dependent plant defense responses. Plant Cell 8:1773-1791. 72. Hammond-Kosack, K. E., and J. D. G. Jones. 1997. Plant disease resistance genes. Annu. Rev. Plant Physiol. Mol. Biol. 48:575-607. 73. Hanekamp, T., D. Kobayashi, S. Hayes, and M. M. Stayton. 1997. Avirulence gene D of Pseudomonas syringae pv. tomato may have undergone horizontal gene transfer. FEBS Lett. 415:4044. 74. Hardt, W. E., and J. E. Galán. 1997. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc. Natl. Acad. Sci. USA 94:9887-9892. 75. Haseloff, J. 1999. GFP variants for multispectral imaging of living cells. Methods Cell Biol. 58:139151. 76. Hassouni, M. E., J. P. Chambost, D. Expert, F. V. Gijsegem, and F. Barras. 1999. The minimal gene set member msrA, encoding peptide methionine sulfoxide reductase, is a virulence determinant of the plant pathogen Erwinia chrysanthemi. Proc. Natl. Acad. Sci. U S A 96:887-892. 77. Hattermann, D. R., and S. M. Ries. 1989. Motility of Pseudomonas syringae pv. glycinea and its role in infection. Phytopathology 79:284-289. 78. He, S. Y., H.-C. Huang, and A. Collmer. 1993. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the hypersensitive response in plants. Cell 73:1255-1266. 79. Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. 80. Hirano, S., A. O. Charkowski, A. Collmer, D. K. Willis, and C. D. Upper. 1999. Role of the Hrp type III protein secretion system in growth of Pseudomonas syringae pv. syringae B728a on host plants in the field. Proc. Natl. Acad. Sci. USA 96:9851-9856. 81. Hirano, S. S., D. I. Rouse, M. K. Clayton, and C. D. Upper. 1995. Pseudomonas syringae pv. syringae and bacterial brown spot of snap bean: a study of epiphytic phytopathogenic bacteria and associated disease. Plant Dis. 79:1085-1093. 82. Hirano, S. S., and C. D. Upper. 1990. Population biology and epidemiology of Pseudomonas syringae. Annu. Rev. Phytopathol. 28:155-177. 25 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 83. Hrabak, E. M., and D. K. Willis. 1993. Involvement of the lemA gene in production of syringomycin and protease by Pseudomonas syringae pv. syringae. Mol. Plant-Microbe Interact. 6:368-375. 84. Huang, H.-C., S. Y. He, D. W. Bauer, and A. Collmer. 1992. The Pseudomonas syringae pv. syringae 61 hrpH product: an envelope protein required for elicitation of the hypersensitive response in plants. J. Bacteriol. 174:6878-6885. 85. Huang, H.-C., S. W. Hutcheson, and A. Collmer. 1991. Characterization of the hrp cluster from Pseudomonas syringae pv. syringae 61 and TnphoA tagging of genes encoding exported or membrane-spanning Hrp proteins. Mol. Plant-Microbe Interact. 4:469-476. 86. Huang, H.-C., R.-W. Lin, C.-J. Chang, A. Collmer, and W.-L. Deng. 1995. The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for harpinPss secretion that are arranged colinearly with Yersinia ysc homologs. Mol. Plant-Microbe Interact. 8:733746. 87. Huang, H.-C., R. Schuurink, T. P. Denny, M. M. Atkinson, C. J. Baker, I. Yucel, S. W. Hutcheson, and A. Collmer. 1988. Molecular cloning of a Pseudomonas syringae pv. syringae gene cluster that enables Pseudomonas fluorescens to elicit the hypersensitive response in tobacco plants. J. Bacteriol. 170:4748-4756. 88. Huang, J., W. Yindeeyoungyeon, R. P. Garg, T. P. Denny, and M. A. Schell. 1998. Joint transcriptional control of xpsR, the unusual signal integrator of the Ralstonia solanacearum virulence gene regulatory network, by a response regulator and a LysR-type transcriptional activator. J. Bacteriol. 180:2736-2743. 89. Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. 90. Hugouvieux-Cotte-Pattat, N., G. Condemine, W. Nasser, and S. Reverchon. 1996. Regulation of pectinolysis in Erwinia chrysanthemi. Annu. Rev. Microbiol. 50:213-257. 91. Huynh, T. V., D. Dahlbeck, and B. J. Staskawicz. 1989. Bacterial blight of soybean: Regulation of a pathogen gene determining host cultivar specificity. Science 245:1374-1377. 92. Innes, R. W., A. F. Bent, B. N. Kunkel, S. R. Bisgrove, and B. J. Staskawicz. 1993. Molecular analysis of avirulence gene avrRpt2 and identification of a putative regulatory sequence common to all known Pseudomonas syringae avirulence genes. J. Bacteriol. 175:4859-4869. 93. Jackson, D. P., D. A. Gray, V. L. Morris, and D. A. Cuppels. 1992. Identification of a DNA region required for growth of Pseudomonas syringae pv. tomato on tomato plants. Can. J. Microbiol. 38:883890. 94. Jackson, R. W., E. Athanassopoulos, G. Tsiamis, J. W. Mansfield, A. Sesma, D. L. Arnold, M. J. Gibbon, J. Murillo, J. D. Taylor, and A. Vivian. 1999. Identification of a pathogenicity island, which contains genes for virulence and avirulence, on a large native plasmid in the bean pathogen Pseudomonas syringae pathovar phaseolicola. Proc. Natl. Acad. Sci. USA 96:10875-10880. 95. Jakobek, J. L., J. A. Smith, and P. B. Lindgren. 1993. Suppression of bean defense responses by Pseudomonas syringae. Plant Cell 5:57-63. 96. Jones, A. J., C. M. Thomas, K. E. Hammond-Kosack, P. J. Balint-Kurti, and J. D. G. Jones. 1994. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 266:789-793. 97. Jones, J. B., J. P. Jones, R. E. Stall, and T. A. Zitter. 1991. Compendium of Tomato Diseases. APS Press, St. Paul. 98. Keen, N. T. 1990. Gene-for-gene complementarity in plant-pathogen interactions. Annu. Rev. Genet. 24:447-463. 99. Keen, N. T., S. Tamaki, D. Kobayashi, D. Gerhold, M. Stayton, H. Shen, S. Gold, J. Lorang, H. Thordal-Christensen, D. Dahlbeck, and B. Staskawicz. 1990. Bacteria expressing avirulence gene D produce a specific elicitor of the soybean hypersensitive reaction. Mol. Plant-Microbe Interact. 3:112121. 100. Kim, J. F., A. O. Charkowski, J. R. Alfano, A. Collmer, and S. V. Beer. 1998. Sequences related to transposable elements and bacteriophages flank avirulence genes of Pseudomonas syringae. Mol. Plant-Microbe Interact. 11:1247-1252. 101. Kim, S. Y., H. J. Chung, and T. L. Thomas. 1997. Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J. 11:1237-1251. 26 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 102. Kitten, T., T. G. Kinscherf, J. L. McEvoy, and D. K. Willis. 1998. A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol 28:917-29. 103. Klenk, H. P., R. A. Clayton, J. F. Tomb, O. White, K. E. Nelson, K. A. Ketchum, R. J. Dodson, M. Gwinn, E. K. Hickey, J. D. Peterson, D. L. Richardson, A. R. Kerlavage, D. E. Graham, N. C. Kyrpides, R. D. Fleischmann, J. Quackenbush, N. H. Lee, G. G. Sutton, S. Gill, E. F. Kirkness, B. A. Dougherty, K. McKenney, M. D. Adams, B. Loftus, J. C. Venter, and et al. 1997. The complete genome sequence of the hyperthermophilic, sulphate- reducing archaeon Archaeoglobus fulgidus. Nature 390:364-370. 104. Klose, K. E., and J. J. Mekalanos. 1998. Distinct roles of an alternative sigma factor during both freeswimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol. Microbiol. 28:501-520. 105. Kost, B., P. Spielhofer, and N. H. Chua. 1998. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 16:393-401. 106. Lawrence, G. J., E. J. Finnegan, M. A. Ayliffe, and J. G. Ellis. 1995. The L6 gene for flax rust resistance is related to the Arabidopsis bacterial resistance gene RPS2 and the tobacco viral resistance gene N. Plant Cell 7:1195-1206. 107. Lawrence, J. G., and H. Ochman. 1998. Molecular archaeology of the Escherichia coli genome. Proc. Natl. Acad. Sci. USA 95:9413-9417. 108. Lazarowitz, S. G., and R. N. Beachy. 1999. Viral movement proteins as probes for intracellular and intercellular trafficking in plants. Plant Cell 11:535-548. 109. Leach, J. E., and F. F. White. 1996. Bacterial avirulence genes. Annu. Rev. Phytopathol. 34:153179. 110. Li, X. Z., A. N. Starratt, and D. A. Cuppels. 1998. Identification of tomato leaf factors that activate toxin gene expression in Pseudomonas syringae pv. tomato DC3000. Phytopathology 88:1094-1100. 111. Lindgren, P. B. 1997. The role of hrp genes during plant-bacterial interactions. Annu. Rev. Phytopathol. 35:129-152. 112. Lindgren, P. B., R. C. Peet, and N. J. Panopoulos. 1986. Gene cluster of Pseudomonas syringae pv. "phaseolicola" controls pathogenicity of bean plants and hypersensitivity on nonhost plants. J. Bacteriol. 168:512-522. 113. Lopez-Solanilla, E., F. Garcia-Olmedo, and P. Rodriguez-Palenzuela. 1998. Inactivation of the sapA to sapF locus of Erwinia chrysanthemi reveals common features in plant and animal bacterial pathogenesis. Plant Cell 10:917-924. 114. Lorang, J. M., H. Shen, D. Kobayashi, D. Cooksey, and N. T. Keen. 1994. avrA and avrE in Pseudomonas syringae pv. tomato PT23 play a role in virulence on tomato plants. Mol. Plant-Microbe Interact. 7:508-515. 115. Lund, S. T., R. E. Stall, and H. J. Klee. 1998. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell 10:371-82. 116. Mahajan, N. P., D. C. Harrison-Shostak, J. Michaux, and B. Herman. 1999. Novel mutant green fluorescent protein protease substrates reveal the activation of specific caspases during apoptosis. Chem. Biol. 6:401-409. 117. Marc, J., C. L. Granger, J. Brincat, D. D. Fisher, T. Kao, A. G. McCubbin, and R. J. Cyr. 1998. A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell 10:1927-1940. 118. Martin, G. B., S. H. Brommonschenkel, J. Chunwongse, A. Frary, M. W. Ganal, R. Spivey, T. Wu, E. D. Earle, and S. D. Tanksley. 1993. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262:1432-1436. 119. Mazzola, M., and F. F. White. 1994. A mutation in the indole-3-acetic acid biosynthesis pathway of Pseudomonas syringae pv. syringae affects growth in Phaseolus vulgaris and syringomycin production. J. Bacteriol. 176:1374-1382. 120. McNellis, T. W., M. B. Mudgett, K. Li, T. Aoyama, D. Horvath, N.-H. Chua, and B. J. Staskawicz. 1998. Glucocorticoid-inducible expression of a bacterial avirulence gene in transgenic Arabidopsis induces hypersensitive cell death. Plant J. 14:247-257. 121. Menard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella entry into epithelial cells. J. Bacteriol. 175:5899-5906. 122. Milligan, S. B., J. Bodeau, J. Yaghoobi, I. Kaloshian, P. Zabel, and V. M. Williamson. 1998. The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10:1307-1319. 27 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 123. Mittal, S., and K. R. Davis. 1995. Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Mol. Plant-Microbe Interact. 8:165-171. 124. Mo, Y.-Y., and D. C. Gross. 1991. Plant signal molecules activate the syrB gene, which is required for syringomycin production by Pseudomonas syringae pv. syringae. J. Bacteriol. 173:5784-5792. 125. Nelson, K. E., R. A. Clayton, S. R. Gill, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, W. C. Nelson, K. A. Ketchum, L. McDonald, T. R. Utterback, J. A. Malek, K. D. Linher, M. M. Garrett, A. M. Stewart, M. D. Cotton, M. S. Pratt, C. A. Phillips, D. Richardson, J. Heidelberg, G. G. Sutton, R. D. Fleischmann, J. A. Eisen, C. M. Fraser, and et al. 1999. Evidence for lateral gene transfer between Archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323-329. 126. Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1-6. 127. O'Donnell, P. J., C. Calvert, R. Atzorn, C. Wasternack, H. M. O. Leyser, and D. J. Bowles. 1996. Ethylene as a signal mediating the wound response of tomato plants. Science 274:1914-1917. 128. Ori, N., Y. Eshed, I. Paran, G. Presting, D. Aviv, S. Tanksley, D. Zamir, and R. Fluhr. 1997. The I2C family from the wilt disease resistance locus I2 belongs to the nucleotide binding, leucine-rich repeat superfamily of plant resistance genes. Plant Cell 9:521-532. 129. Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. 130. Pallen, M. J. 1999. Microbial genomes. Mol. Microbiol. 32:907-912. 131. Palmer, D. A., and C. L. Bender. 1995. Ultrastructure of tomato leaf tissue treated with the Pseudomonad phytotoxin coronatine and comparison with methyl jasmonate. Mol. Plant-Microbe Interact. 8:683-692. 132. Panopoulos, N. J., and M. N. Schroth. 1974. Role of flagellar motility in the invasion of bean leaves by Pseudomonas phaseolicola. Phytopathology 64:1389-1397. 133. Pautot, V., F. M. Holzer, and L. L. Walling. 1991. Differential expression of tomato proteinase inhibitor I and II genes during bacterial pathogen invasion and wounding. Mol. Plant-Microbe Interact. 4:284-292. 134. Pontier, D., M. Tronchet, P. Rogowsky, E. Lam, and D. Roby. 1998. Activation of hsr203, a plant gene expressed during incompatible plant- pathogen interactions, is correlated with programmed cell death. Mol. Plant Microbe. Interact. 11:544-554. 135. Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. 136. Preston, G., W.-L. Deng, H.-C. Huang, and A. Collmer. 1998. Negative regulation of hrp genes in Pseudomonas syringae by HrpV. J. Bacteriol. 180:4532-4537. 137. Pugsley, A. P., O. Francetic, O. M. Possot, N. Sauvonnet, and K. R. Hardie. 1997. Recent progress and future directions in studies of the main terminal branch of the general secretory pathway in Gramnegative bacteria - a review. Gene 192:13-19. 138. Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899-1902. 139. Richael, C., and D. Gilchrist. 1999. The hypersensitive response: A case of hold or fold? Physiol. Mol. Plant Pathol. 55:5-12. 140. Riley, M. 1993. Functions of the gene products of Escherichia coli. Microbiol. Rev. 57:862-652. 141. Rohde, B. H., B. Pohlack, and M. S. Ullrich. 1998. Occurrence of thermoregulation of genes involved in coronatine biosynthesis among various Pseudomonas syringae strains. J Basic Microbiol 38:41-50. 142. Roine, E., D. N. Nunn, L. Paulin, and M. Romantschuk. 1996. Characterization of genes required for pilus expression in Pseudomonas syringae pathovar phaseolicola. J. Bacteriol. 178:410-417. 143. Ronald, P. C., J. M. Salmeron, F. M. Carland, and B. J. Staskawicz. 1992. The cloned avirulence gene avrPto induces disease resistance in tomato cultivars containing the Pto resistance gene. J. Bacteriol. 174:1604-1611. 144. Rossi, M., F. L. Goggin, S. B. Milligan, I. Kaloshian, D. E. Ullman, and V. M. Williamson. 1998. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. U S A 95:9750-9754. 145. Rudolph, K., T. J. Burr, J. W. Mansfield, D. Stead, A. Vivian, and J. von Kietzell. 1997. Pseudomonas syringae Pathovars and Related Pathogens. Kluwer, Dordrecht. 28 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 146. Salmeron, J. M., G. E. D. Oldroyd, C. M. T. Rommens, S. R. Scofield, H.-S. Kim, D. T. Lavelle, D. Dahlbeck, and B. J. Staskawicz. 1996. Tomato Prf is a member of the leucine-rich repeat class of plant disease resistance genes and lies embedded within the Pto kinase gene cluster. Cell 86:123133. 147. Salzberg, S. L., A. L. Delcher, S. Kasif, and O. White. 1998. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 26:544-548. 148. Sanderfoot, A. A., and S. G. Lazarowitz. 1996. Getting it together in plant virus movement: cooperative interactions between bipartite geminivirus movement proteins. Cell Biology 6:353-358. 149. Satiat-Jeunemaitre, B., P. Boevink, and C. Hawes. 1999. Membrane trafficking in higher plant cells: GFP and antibodies, partners for probing the secretory pathway. Biochimie 81:597-605. 150. Schaefer, B. C. 1995. Revolutions in rapid amplification of cDNA ends: new strategies for polymerase chain reaction cloning of full-length cDNA ends. Anal. Biochem. 227:255-273. 151. Schaller, A., and C. A. Ryan. 1996. Systemin--a polypeptide defense signal in plants. Bioessays 18:27-33. 152. Scofield, S. R., C. M. Tobias, J. P. Rathjen, J. H. Chang, D. T. Lavelle, R. W. Michelmore, and B. J. Staskawicz. 1996. Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274:2063-2065. 153. Sharma, S. B., and E. R. Signer. 1990. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Develop. 4:344-356. 154. Shen, H., and N. T. Keen. 1993. Characterization of the promoter of avirulence gene D from Pseudomonas syringae pv. tomato. J. Bacteriol. 175:5916-5924. 155. Simons, G., J. Groenendijk, J. Wijbrandi, M. Reijans, J. Groenen, P. Diergaarde, T. Van der Lee, M. Bleeker, J. Onstenk, M. de Both, M. Haring, J. Mes, B. Cornelissen, M. Zabeau, and P. Vos. 1998. Dissection of the fusarium I2 gene cluster in tomato reveals six homologs and one active gene copy. Plant Cell 10:1055-1068. 156. Song, W.-Y., G.-L. Wan, L.-L. Chen, H.-S. Kim, L.-Y. Pi, T. Holsten, J. Gardner, B. Wang, W.-X. Qhai, L.-H. Zhu, C. Fauquet, and P. Ronald. 1995. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270:1804-1806. 157. Suoniemi, A., K. Bjorklof, K. Haahtela, and M. Romantschuk. 1995. Pili of Pseudomonas syringae pathovar syringae enhance initiation of bacterial epiphytic colonization. Microbiology 141:497-503. 158. Sutton, G. G., O. White, M. D. Adams, and A. R. Kerlavage. 1995. TIGR Assember: A new tool for assembling large shotgun sequencing projects. Genome Seq. Technol. 1:9-19. 159. Swords, K. M. M., D. Dahlbeck, B. Kearney, M. Roy, and B. J. Staskawicz. 1996. Spontaneous and induced mutations in a single open reading frame alters both virulence and avirulence in Xanthomonas campestris pv. vesicatoria avrBs2. J. Bacteriol. 178:4661-4669. 160. Tai, T. H., D. Dahlbeck, E. T. Clark, P. Gajiwala, R. Pasion, M. C. Whalen, R. E. Stall, and B. J. Staskawicz. 1999. Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc. Natl. Acad. Sci. U S A 96:14153-14158. 161. Tang, X., R. D. Frederick, J. Zhou, D. A. Halterman, Y. Jia, and G. B. Martin. 1996. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274:2060-2062. 162. Tao, H., C. Bausch, C. Richmond, F. R. Blattner, and T. Conway. 1999. Functional genomics: expression analysis of Escherichia coli growing on minimal and rich media. J. Bacteriol. 181:64256440. 163. Thomas, C. M., D. J. Jones, M. Parniske, K. Harrison, P. J. Balint Kurti, K. Hatzixanthis, and J. D. G. Jones. 1997. Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. Plant Cell 9:2209-2224. 164. Thomashow, L. S. 1996. Biological control of plant root pathogens. Curr. Opin. Biotechnol. 7:343347. 165. Thoquet, P., J. Olivier, C. Sperisen, P. Rogowsky, H. Laterrot, and N. Grimsley. 1996. Quantitative trait loci determining resistance to bacterial wilt in tomato cultivar Hawaii 7996. Mol. Plant-Microbe Interact. 9:826-836. 166. Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, and et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. 29 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 167. Tornero, P., J. Gadea, V. Conejero, and P. Vera. 1997. Two PR-1 genes from tomato are differentially regulated and reveal a novel mode of expression for a pathogenesis-related gene during the hypersensitive response and development. Mol. Plant-Microbe Interact. 10:624-634. 168. Valent, B., G. T. Bryan, Y. Jia, L. Farrall, K. Wu, H. P. Hershey, S. A. McAdams, R. Tarchini, K. Faulk, and G. Donaldson. 2000. Molecular characterization of resistance gene/avirulence gene interactions in the rice blast system, p. In P. J. G. M. de Wit, T. Bisseling, and W. Stiekema (ed.), Biology of Plant-Microbe Interactions, vol. 2. International Society for Molecular Plant-Microbe Interactions, 169. van Dijk, K., D. E. Fouts, A. H. Rehm, A. R. Hill, A. Collmer, and J. R. Alfano. 1999. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J. Bacteriol. 181:4790-4797. 170. Van Gijsegem, F. 1997. In planta regulation of phytopathogenic bacteria virulence genes: relevance of plant-derived signals. Eur. J. Plant Pathol. 103:291-301. 171. van Helden, J., B. Andre, and J. Collado-Vides. 1998. Extracting regulatory sites from the upstream region of yeast genes by computational analysis of oligonucleotide frequencies. J. Mol. Biol. 281:827842. 172. Vera-Estrella, R., E. Blumwald, and V. J. Higgins. 1992. Effect of specific elicitors of Cladosporium fulvum on tomato suspension cells. Plant Physiol. 99:1208-1215. 173. Vivian, A., M. J. Gibbon, and J. Murillo. 1997. The molecular genetics of specificity determinants in plant pathogenic bacteria, p. 293-328. In I. R. Crute, E. B. Holub, and J. J. Burdon (ed.), The Genefor-Gene Relationship. CAB International, 174. Vos, P., G. Simons, T. Jesse, J. Wijbrandi, L. Heinen, R. Hogers, A. Frijters, J. Groenendijk, P. Diergaarde, M. Reijans, J. Fierens-Onstenk, M. de Both, J. Peleman, T. Liharska, J. Hontelez, and M. Zabeau. 1998. The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat. Biotechnol. 16:1365-1369. 175. Wanner, L. A., and W. Gruissem. 1991. Expression dynamics of the tomato rbcS gene family during development. Plant Cell 3:1289-1303. 176. Warren, R. F., P. M. Merritt, E. Holub, and R. W. Innes. 1999. Identification of three putative signal transduction genes involved in R gene-specified disease resistance in Arabidopsis. Genetics 152:401-412. 177. Waterman, M. S. 1988. Computer analysis of nucleic acid sequences. Methods Enzymol. 164:765773. 178. Watterson, J. C. 1986. Diseases, p. 443-484. In (ed.), The Tomato Crop - A Scientific Basis for Improvement. Chapman and Hall Ltd, London. 179. Whalen, M. C., R. W. Inness, A. F. Bent, and B. J. Staskawicz. 1991. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3:49-59. 180. Wharam, S. D., V. Mulholland, and G. P. C. Salmond. 1995. Conserved virulence factor regulation and secretion systems in bacterial pathogens of plants and animals. Eur. J. Plant Pathol. 101:1-13. 181. White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, R. J. Dodson, D. H. Haft, M. L. Gwinn, W. C. Nelson, D. L. Richardson, K. S. Moffat, H. Qin, L. Jiang, W. Pamphile, M. Crosby, M. Shen, J. J. Vamathevan, P. Lam, L. McDonald, T. Utterback, C. Zalewski, K. S. Makarova, L. Aravind, M. J. Daly, C. M. Fraser, and et al. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. 182. Whitham, S., S. P. Dinesh-Kumar, D. Chol, R. Hehl, C. Corr, and B. Baker. 1994. The product of the tobacco mosaic virus resistance gene N: similarity to toll and interleukin-1 receptor. Cell 78:11011115. 183. Wilkinson, J. Q., M. B. Lanahan, H. C. Yen, J. J. Giovannoni, and H. J. Klee. 1995. An ethyleneinducible component of signal transduction encoded by Never-ripe. Science 270:1807-1809. 184. Willis, D. K., E. M. Hrabak, J. J. Rich, T. M. Barta, S. E. Lindow, and N. J. Panopoulos. 1990. Isolation and characterization of a Pseudomonas syringae pv. syringae mutant deficient in lesion forming ability on bean. Mol. Plant-Microbe Interact. 3:149-156. 30 Functional Genomics of the Interactions of Tomato and Pseudomonas syringae pv tomato DC3000 A. Collmer, PI 185. Willis, D. K., J. J. Rich, T. G. Kinscherf, and T. Kitten. 1994. Genetic regulation in plant pathogenic pseudomonads, p. 167-193. In J. K. Setlow (ed.), Genetic Engineering, Vol. 16. Plenum Press, New York. 186. Wilson, M., J. DeRisi, H. H. Kristensen, P. Imboden, S. Rane, P. O. Brown, and G. K. Schoolnik. 1999. Exploring drug-induced alterations in gene expression in Mycobacterium tuberculosis by microarray hybridization. Proc. Natl. Acad. Sci. USA 96:12833-12838. 187. Winans, S. C., D. L. Burns, and P. J. Christie. 1996. Adaptation of a conjugal transfer system for export of pathogen macromolecules. Trends Microbiol. 4:64-68. 188. Wubben, J. P., C. B. Lawrence, and P. J. G. M. de Wit. 1996. Differential induction of chitinase and 1,3-b-glucanase gene expression in tomato by Cladosporium fulvum and its race-specific elicitors. Physiol. Mol. Plant Pathol. 48:105-116. 189. Xiao, Y., S. Heu, J. Yi, Y. Lu, and S. W. Hutcheson. 1994. Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J. Bacteriol. 176:1025-1036. 190. Xiao, Y., and S. Hutcheson. 1994. A single promoter sequence recognized by a newly identified alternate sigma factor directs expression of pathogenicity and host range determinants in Pseudomonas syringae. J. Bacteriol. 176:3089-3091. Author's correction. 176:6158. 191. Xiao, Y., Y. Lu, S. Heu, and S. W. Hutcheson. 1992. Organization and environmental regulation of the Pseudomonas syringae pv. syringae 61 hrp cluster. J. Bacteriol. 174:1734-1741. 192. Young, S. A., X. Wang, and J. E. Leach. 1996. Changes in the plasma membrane distribution of rice phospholipase D during resistant interactions with Xanthomonas oryzae pv oryzae. Plant Cell 8:10791090. 193. Yu, I. C., J. Parker, and A. F. Bent. 1998. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc. Natl. Acad. Sci. USA 95:7819-7824. 194. Yu, J., A. Penaloza-Vazquez, A. M. Chakrabarty, and C. L. Bender. 1999. Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Mol. Microbiol. 33:712-720. 195. Zhang, Z., A. A. Schaffer, W. Miller, T. L. Madden, D. J. Lipman, E. V. Koonin, and S. F. Altschul. 1998. Protein sequence similarity searches using patterns as seeds. Nucleic Acids Res. 26:39863990. 196. Zhou, J., Y.-T. Loh, R. A. Bressan, and G. B. Martin. 1995. The tomato gene Pti1 encodes a serine/threonine kinase that is phosphorylated by Pto and is involved in the hypersensitive response. Cell 83:925-935. 197. Zhou, J., X. Tang, and G. B. Martin. 1997. The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. EMBO J 16:3207-3218. 198. Zhu, W., B. Yang, J. M. Chittoor, L. B. Johnson, and F. F. White. 1998. AvrXa10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol. Plant-Microbe Interact. 11:824-32. 31