Form 45 Viral Vaccine (jan2009
advertisement

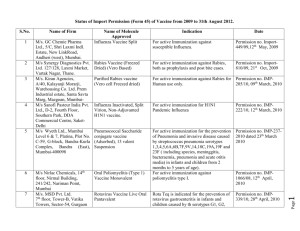
(Form-45) Import Permission issued from January 2009 to December 2010 for Viral Vaccines S.No. Name of the Firm Name of the Product Composition Indication Date of Permision 1 Nirlac Chemicals Oral Poliomyelitis (Type 1) Vaccine Monovalent Each 0.1 ml dose (2 drops) contains:-Live attenuated poliomyelitis virus type 1(Sabin Strain) ≥106.0CCID 50, Erythromycin ≤ 2mcg, Kanamycin ≤10mcg, Sucrose - 35% v/v. Active immunization against poliomyelitis type 1 12 April, 2010 2 M/s. Kiran Agencies Purified rabies vaccine (Vero cell Freezed dried) For active Immunization angainst rabies 09th March, 2010 3 M/s. MSD Rotavirus Vaccine Live Oral Pantavalent Each Single dose 0.5 ml Vials contains after Reconstitution with Diluent contains- rabies Virus (PV 2061 strain) ≥2.5 IU Each single dose of 2mL contains: - (API) G1 Reassortant - 2.2 ×106 IU/dose, G2 Reassortant - 2.8 ×106 IU/dose, G3 Reassortant - 2.2 ×106 IU/dose, G4 Reassortant - 2.0 ×106 IU/dose, P1 Reassortant - 2.3 ×106 IU/dose, (Inactive Ingredient) Sucrose – 1080mg, Sodium citrate Dihydrate – 127mg, Sodium phosphate Monobasic Monohydrate 29.8mg, Sodium Hydroxide - 2.75mg, Polysorbate – 80 0.17 – 0.86mg, Rotavirus Diluent & LPKM-3 - 15% (v/v) For the prevention of rotavirus gastroenteritis in infants and children caused by the serotypes G1, G2, G3 and G4 when administered as a 3 dose series to infants between 6 to 32 weeks. The first dose of Rota Teq should be administered between 6 and 12 weeks of age 28th April, 2010 4 Synergy Diagnostics Pvt. Ltd. rabies Vaccine Freezed Dried Vero Based Each 0.5 ml contains:- Rabies Antigen equivalent to ≥2.5IU For active immunization against Rabies, Both as prophylaxis and post bite cases For active immunization against susceptible Influenzae 21th Oct, 2009 5 M/s. GC Chemie Pharma Ltd. Influenza Vaccine Split Each 0.5 ml vial /PFS contains: A/Brisbane/59/2007 (H1N1) like virus --15mcg ,A/Brisbane/10/2007 (H3N2) like virus ---15 mcg ,B/Florida/4/2006 like virus ------15 mcg 6 Serum Institute of India Inactivated Polio Vaccine (Type 1, 2, &3) Bulk Inactivated Polio Vaccine (Type 1, 2, &3) Bulk Active immunization against poliomyelitis (Type 1, 2, &3) 17th Feb, 2009 7 Sanofi Pasteur India Pvt. Ltd., Delhi Influenza Inactivated, Split Virion, NonAdjuvanted H1N1 vaccine. 15µg of HA/Human dose For active immunization for H1N1 pandemic influenza 12th March, 2010 8 Ranbaxy Laboratories Purified rabies vaccine (Vero cell Freezed dried) Each Single Dose(0.5ml) vial after reconstituted with diluents contains : Rabies Virus (PV2061 Strain)Cultured on Vero Cell Inactivated) ≥2.5IU For active immunization against Rabies for Human use only. 22th Sep, 2010 12 May, 2009