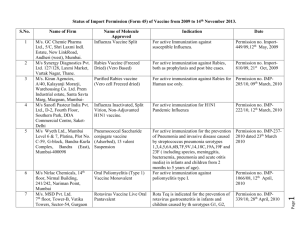
Status of Import Permission (Form 45) of Vaccine from 2009 to 31th
advertisement

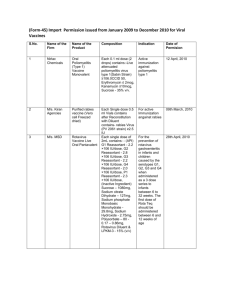
Status of Import Permission (Form 45) of Vaccine from 2009 to 31th August 2012. 1 M/s. GC Chemie Pharma Ltd., 5/C, Shri Laxmi Indl. Estate, New LinkRoad, Andheri (west), Mumbai. M/s Synergy Diagnostics Pvt. Ltd. 127/128, Laxmi Market, Vartak Nagar, Thane. M/s. Kiran Agencies, A/40, Kalayanji Morarji, Warehousing Co. Ltd. Prem Industrial estate, Santa Savta Marg, Mazgoan, MumbaiM/s Sanofi Pasteur India Pvt. Ltd., D-2, Fourth Floor, Southern Park, DDA Commercial Centre, SaketDelhi M/s Wyeth Ltd., Mumbai Level 6 & 7, Platina, Plot No. C-59, G-block, Bandra-Kurla Complex, Bandra (East), Mumbai-400098 2 3 4 5 6 7 M/s Nirlac Chemicals, 14th floor, Nirmal Building, 241/242, Nariman Point, Mumbai M/s. MSD Pvt. Ltd. 7th floor, Tower-B, Vatika Towers, Sector-54, Gurgaon Name of Molecule Approved Influenza Vaccine Split Indication Date For active Immunization against susceptible Influenza. Permission no. Import449/09,12th May, 2009 Rabies Vaccine (Freezed Dried) (Vero Based) For active immunization against Rabies, both as prophylaxis and post bite cases. Permission no. Import810/09, 21st Oct, 2009 Purified Rabies vaccine (Vero cell Freezed dried) For active immunization against Rabies for Human use only. Permission no. IMP205/10, 09th March, 2010 Influenza Inactivated, Split Virion, Non-Adjuvanted H1N1 vaccine. For active immunization for H1N1 Pandemic Influenza Permission no. IMP222/10, 12th March, 2010 Pneumococcal Saccharide conjugate vaccine (Adsorbed), 13 valent Suspension For active immunization for the prevention of Pneumonia and invasive disease caused by streptococcus pneumonia serotypes 1,3,4,5,6A,6B,7F,9V,14,18C,19A,19F and 23F ( including species, meninggitis, bacteraemia, pneumonia and acute otitis media) in infants and children from 2 months to 5 years of age). For active immunization against poliomyelitis type I. Permission no. IMP-2372010 dated 23th March 2010 Rota Teq is indicated for the prevention of rotavirus gastroenteritis in infants and children caused by th serotypes G1, G2, Permission no. IMP339/10, 28th April, 2010 Oral Poliomyelitis (Type 1) Vaccine Monovalent Rotavirus Vaccine Live Oral Pantavalent Permission no. IMP1066/08, 12th April, 2010 1 Name of Firm Page S.No. Purified Rabies vaccine (Vero cell Freezed dried) 10 M/s GlaxoSmithKline Pharmaceuticals Ltd., Mumbai Synflorix [Pneumococcal polysaccharide and NonTypeable Haemophilus influenzae(NTHi) protein D conjugate vaccine, adsorbed (10 Pn-PD-DiT)] 11 M/s Novartis Healthcare Pvt. Ltd., Sandoz House, Dr. Annie Besant Road, Worli, Mumbai. M/s Sanofi Pasteur India Pvt. Ltd., 54/A, Sir Mathuradas Vasanji Road, Andheri East, Mumabi-400093 Recombinant Hepatitis-B Vaccine (Sci-B-Vac) from 9 12 Purified Rabies vaccine (vero For active immunization against Rabies for cell ) for Human Use Freeze Human use only. dried Meningococcal A C Y and W-135 Polysaccharide Diphtheria Toxoid Conjugate Permission no. IMP647/2010, 22nd Sep, 2010 Permission no. IMP94/2011, 22nd March 2011 Active immunization of all infants and children from 6 weeks up to 5 years of age against diseases caused by Streptococcus pneumonia serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23f (including sepsis, meningitis, pneumonia, bacteraemia and acute otitis media) and against acute otitis media caused by Non Typeable Haemophilus influenzae. For active immunization against Hepatitis B virus. Permission no. IMP102/2011, 20th Apr 2011 For active immunization of healthy 2 years to 55 years age group for prevention of invasive meningococcal disease caused by N.meningitides serogroups A, C, Y, and W-135. Permission No. IMP134/2012 dated 12th June 2012 Permission no. IMP254/11, 2nd June 2011 2 M/s Ranbaxy Laboratories Ltd., Plot No. 90, Sector 32, Gurgaon M/s HLL Life Care Ltd.(formerly Hindustan Latex Limited), HILL Bhavan, Pooojappura, Thiruvananthapuram, Kerala Page 8 G3 and G4 when administered as a 3 dose series to infants between 6 to 32 weeks. The first dose of Rota Teq should be administered between 6 to 12 weeks of age. For active immunization against Rabies for Human use only.