antimicrobial activities of sulfur compounds derived from s
advertisement

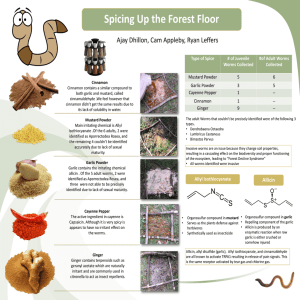
ANTIMICROBIAL ACTIVITIES OF SULFUR COMPOUNDS DERIVED FROM S-ALK(EN)YL-L-CYSTEINE SULFOXIDES IN ALLIUM AND BRASSICA K. H. Kyung1 and Y. C. Lee2 1 Dept. of Food Sci., Sejong Univ., Seoul 143-747, Korea 2 Dept. of Food Sci. & Technol., Chung-Ang Univ., Ansung 456-756, Korea ABSTRACT Allium and Brassica vegetables have long been known for their antimicrobial activity against various microorganisms, including Gram-positive and Gram-negative bacteria and fungi. Most of microorganisms tested were sensitive to extracts of the Allium and Brassica vegetables and the degree of sensitivity varied depending on the strain under study and test conditions. Among the vegetables, garlic showed the most potent activity, followed by onion. Brassica including cabbage showed the least potent activity. The principal antimicrobial compounds of Allium and Brassica have been elucidated as allicin (S-allyl-Lpropenethiosulfinate) and methyl methanethiosulfinate, respectively. Both compounds belong to the same chemical group, thiosulfinate, generated from S-allyl and S-methyl derivatives of L-cysteine sulfoxide, respectively, existing in Allium and Brassica as major non-protein sulfur containing amino acids. There have been only few applications of garlic as a natural food preservative, in spite of numerous studies on antimcirobial activity of the vegetables. Relative instability of the antimicrobial compounds and strong odor of their mother plants seem to limit the use of them as a practical food preservative. KEY WORDS: Antimicrobial activity, S-alk(en)yl-L-cysteine sulfoxide, garlic, cabbage, Allium and Brassica INTRODUCTION The antimicrobial activities of plant extracts, especially from Allium and Brassica have been recognized for many years, since Walton et al. (1) and Sherman and Hodge (2) scientifically demonstrated the presence of antimicrobial activity of garlic (Allium sativum) and cabbage (Brassica oleracae), respectively. The major antimicrobial activity of Allium (3,4) and Brassica (5) vegetables is proven to be due to volatile sulfur compounds derived from S-alk(en)yl-L-cysteine sulfoxide, as well as glucosinolates in case of Brassica (6-8). The presence of S-alkenyl-L-cysteine sulfoxides is confined essentially to two families; the Cruciferae and the Lilliaceae where they are particularly associated with Allium and Brassica (9). The antimicrobial activity of garlic has been the most studied area for natural antimicrobials. Cavallito and Bailey (3) succeeded in isolating allicin (allyl 2-propenethiosulfinate) which is absent in intact garlic, but generated from its precursor, alliin (S-allyl-L-cysteine sulfoxide), through enzymatic hydrolysis when the tissue of garlic is disrupted (3, 4, 10). Although relatively fewer research has been conducted on the antimicrobial activity of cabbage compared with garlic, the presence of antimicrobial activity of Brassica including cabbage, has been confirmed (5, 11-14) following the initial demonstration of the activity in 1936 by Sherman and Hodge (2). S-Methyl-L-cysteine sulfoxide (SMCSO), a non-protein sulfur-containing amino acid in Brassica (15-17), is structurally similar to alliin, another example of non-protein amino acid commonly found in Allium including garlic. SMCSO and alliin are methyl and allyl derivatives of L-cysteine sulfoxide, respectively. The two compounds generate methyl methanethiosulfinate (MMTSO) and allicin, which are principal antimicrobial compounds of Brassica and Allium, respectively, as a result of enzyme reaction when the tissue of vegetables is disrupted. MMTSO and allicin have similar chemical structure, and are methyl and allyl derivatives of thiosulfinate. Some other genera among Cruciferae, Synapis (S. alba L.; mustard), Raphanus (R. sativus; radish), Cherianthus (C. cheiri; wallflower), and Capsell (C. bursa-pastoris L.; shepherd's purse) also contain SMCSO (15). Although Allium and Brassica vegetables have been reported to have other biological activities, such as cancer-preventive (17, 18, 20, 21), anti-ulcerative (22), serum lipidlowering (23), antiviral (24), antithrombotic (25), hemolytic anaemia-causing (26, 27) and enzyme inhibitory (28-32) activities, this article reviews the chemistry of S-alk(en)yl-L-cysteine-sulfoxides of Allium and Brassica and the antimicrobial activity of sulfur compounds derived from them. ANTIMICROBIAL ACTIVITY OF EXTRACTS OF ALLIUM AND BRASSICA VEGETABLES Antimicrobial activity of garlic extract has been recognized for many years. It was reported that 1-2% garlic extract inhibited microbial growth, and higher concentrations were germicidal. Dababneh and Al-Delaimy (33) reported that 1% garlic extract inhibited Staphylococcus aureus. Karaioannoglou et al. (34) indicated that garlic extract >1% in culture media was inhibitory to Lactobacillus plantarum, and >2% was bactericidal. Mantis et al. (35) showed that 2% garlic extract in culture media had growth inhibitory effects against S. aureus. Extracts of <1% were non-inhibitory and those of >5% were germicidal. Garlic extract at 1% was strongly inhibitory against yeasts (36). Salmonella typhimurium (37), Bacillus cereus (38), Clostridium botulinum (39), C. perfringens (40), Candida utilis (24, 41) and many other bacteria (24, 42, 43) and fungi (24, 44, 45) have been inhibited by garlic extract. S. aureus, Escherichia coli, Proteus mirabilis and Pseudomonas aeruginosa which are multiple resistant to antibiotics including penicillin, streptomycin, doxycilline and cephalexin were inhibited by garlic extract (46). A multiple resistant Klebsiella sp. was not inhibited by garlic extract. hin et al. (47) evaluated the antimicrobial activity of garlic juice powder dehydrated by different methods. Freeze-dried garlic juice powder showed high inhibitory effect on Gram positive bacteria (Bacillus subtilis, S. aureus and Streptococcus mutans) having 0.3 2.0% minimum inhibitory concentrations (MIC). But spray-dried garlic juice powder did not have inhibitory effect on Gram positive bacteria, except B. subtilis. Garlic juice powder processed with both hydration methods did not show any difference in inhibitory effects on E. coli and E. coli O 157:H7 with 1.0 2.0%. The antimicrobial activity of onion is relatively weaker than that of garlic and an area of fewer research. While 1-4% garlic extract completely inhibited E. coli, S. typhosa, Shigella dysenterie and S. aureus, 4% onion extract completely inhibited the growth of only S. dysenterie and S. aureus. (42). However, in a test with non-growing S. typhimurium, freshly reconstituted dehydrated onion showed a stronger bactericidal activity compared with freshly reconstituted dehydrated garlic (37). The presence of the antimicrobial activity in cabbage is definite (5, 11-13, 48, 49), but much less potent compared with those of garlic and onion. The antimicrobial activity of cabbage was reported to be destroyed by heating (2, 11) as is the case with garlic. Yildiz and Westhoff (49), however, reported that heating caused cabbage extract to become inhibitory. Kyung and Fleming (5) demonstrated inhibitory activity in fresh, unheated juice of several cultivars of cabbage. Heating the cabbage before juice extraction prevented formation of the inhibitor(s) in some cultivars, but not others. The growth inhibitory substance of fresh cabbage was suggested to be carbohydrate in nature and of low molecular weight (13, 14). The identity of the inhibitory compound has recently been elucidated as MMTSO generated from SMCSO in cabbage (48). S-ALK(EN)YL-L-CYSTEINE SULFOXIDES; THE PRECURSORS Brassica is taxonomically far apart from the genus Allium which includes garlic and onion. However, they have in common, that S-alk(en)yl-L-cysteine sulfoxides as major non-protein amino acids (50). S-Alk(en)ylL-cysteine sulfoxides were suggested to be important in sulfur metabolism, acting as a soluble pool for organic sulfur (51). The amounts vary widely depending on plant species and on different parts of the plants (16, 48). The general structure of the S-alk(en)yl-L-cysteine sulfoxides is shown in Fig. 1. Five S-alk(en)ylL-cysteine sulfoxides differing in R-side group have been described; four in Allium and one in Brassica. Variety of R-side group of S-alk(en)yl-L-cysteine sulfoxides together with some source plants and their amounts are given in Table 1. Figure 1. Enzymatic cleavage of S-alk(en)yl-L-cysteine sulfoxides Rundqvist (52) made the first effort to isolate the basic principle from which volatile sulfur compounds are generated in garlic. Owing to the fact that the precipitation he obtained contained considerable quantities of carbohydrate, he thought that the compound he was seeking was a glucoside which he named "alliin". The term alliin has since been used (4). Later, pure alliin has been isolated by Stoll and Seebeck (53) and identified as an amino acid. Alliin was chemically synthesized in 1951 by the same group(54). SMCSO was initially isolated from cruciferous vegetables in two separate laboratories at the approximately same time (15, 16). They analyzed SMCSO in cruciferous vegetables among which Brassica contained it invariably, while other genera in Cruciferae showed mixed results. Table 1. Kinds and amounts of S-alk(en)yl-L-cysteine sulfoxides in Allium and Brassica*. Genus Alk(en)yl group Plant sources and amounts of S-alk(en)yl-L-cysteine sulfoxides(mg/kg) in parentheses Allium methyl garlic, elephant garlic, wild garlic, onion [200 (9)], leek, scallion, shallot, Chinese chive propyl onion [50 (9)], leek, scallion, shallot, chive propenyl garlic, elephant garlic, wild garlic, onion [40* (9), leek, scallion, shallot, Chinese chive, chive allyl garlic [900-11500 (54)], elephant garlic, wild garlic, Chinese chive methyl cabbage [185-2218 (15-17, 47, 60)], kale [1310-1380 (26)], turnip[43-202 (16)], swede, Chinese Brassica cabbage [396-786 (16, 50)], cauli-flower [2380 (16)], kohlrabi [558-1069 (16)], broccoli [3432406(16, 61)] From Block et al. (10). When S-alk(en)yl-L-cysteine sulfoxides of garlic were analyzed by reverse-phase HPLC, S-methyland S-allyl-L-cysteine sulfoxides were the only compounds which were identified with certainty (55). Other sulfur amino acids were not found. Ziegler & Sticher (55) opined that the minor derivatives of Lcysteine sulfoxide were absent or were below detection limits under the chromatographic conditions. From the GC analytical results of thiosulfinates of crushed garlic, Freeman and Whenham (56) showed that the L-cysteine sulfoxide fraction of garlic consists of 85% alliin along with 2% S-propyl cysteine sulfoxide and 13% SMCSO. Therefore it can be safely assumed that the concentration of minor Lcysteine sulfoxides could be too low to be detected by HPLC by Ziegler and Sticher (55). Block et al. (10) reported HPLC analytical results of thiosulfinates of garlic and showed that their ratios of allyl/methyl were similar to that of Freeman and Whenham (56). The allyl/methyl ratio of garlic (10) ranged from 94:2 (New York grown) to 80:16 (store bought garlic) to 74:24 (Indian garlic grown at 32℃). Occurrence of ethyl (57, 58), and propyl (56) and butyl (58) derivatives in garlic and allyl derivatives in onion (59) was suggested, but has never been positively confirmed since (55). Allyl groups are absent in onion, scallion, shallot, leek, and chive and propyl groups are absent in garlic, elephant garlic, wild garlic and Chinese chive (10; Table 1), judging from various thiosulfinates isolated from Allium plants (Table 2). Table 2. Thiosulfinates from the extracts of Allium and Brassica Allium and Brassica vegetables Thiosulfinates AllSS(O)propenyl-(E): garlic, elephant garlic AllS(O)Spropenyl-(Z,E): garlic, elephant garlic, wild garlic AllS(O)Sall: garlic, elephant garlic, wild garlic n-pro-SS(O)propenyl-(E): onion, shallot, scallion, leek, chive n-pro-S(O)S-propenyl-(Z,E): onion, shallot, scallion, leek, chive n-pro-S(O)S-pro-n: onion, shallot, scallion, leek, chive Me-S(O)S-Me: onion, shallot, scallion, leek, garlic, all Brassica All-S(O)S-Me: garlic, elephant garlic, wild garlic, Chinese chive Me-S(O)S-propenyl-(Z,E): onion, shallot, scallion, leek, garlic, elephant garlic, wild garlic, Chinese chive, chive Me-SS(O)-pr: onion, shallot, scallion, leek, chive Me-S(O)S-pr: onion, shallot, scallion, leek, chive All-SS(O)-Me: garlic, elephant garlic, wild garlic, Chinese chive Me-S(O)S-Me: onion, shallot, scallion, leek, garlic, elephant garlic, wild garlic, Chinese chive, chive From Block et al. (1992). For Brassica, Marks et al. 1992 The concentration of the minor unsymmetrical thiosulfinates, which possess 1-propenyl group, varied with the age and storage condition of garlic following harvesting. 1-Propenyl levels increased upon refrigeration (10). All the Brassica vegetables including kale, swede, turnip, cabbage, Chinese cabbage, cauliflower, kohlrabi and broccoli contains only S-methyl- derivative of L-cysteine sulfoxide (16; Table 1). Onion contained γ-L-glutamyl-(+)-S-propenyl-L-cysteine sulfoxide and cycloalliin in addition to propyl, methyl, propenyl derivatives of L-cysteine sulfoxide. Cycloalliin could be an artefact during elution from cationic exchange resin, since S-propenyl-L-cysteine sulfoxide spontaneously cyclizes at pH> 7 (9). Growth condition is known to modify the profile of S-alkenyl-L-cysteine sulfoxides of Allium and Brassica. Some garlic varieties grown in cooler climates show a higher allyl to methyl ratio than garlic grown in warmer climates (10). For example, NY grown garlic revealed low levels of (+)S-methyl-L-cysteine sulfoxide (0.08-0.25mg/g of garlic compared to 1-1.6mg/g in California garlic) with normal levels of other derivatives (10). Block et al.(10) suggested that garlic grown in colder climates are subject to stress and that this stress causes reduced synthesis of SMCSO. Cruciferous vegetable species like kale, however, has been known to accumulate more SMCSO when grown during periods of frost (60, 61). ENZYMES AND THEIR REACTION PRODUCTS Enzymes In Allium plants, Alliin and alliinase are located in different compartments (63); the substrates in the cytoplasm and enzyme in the vacuole. The reaction of alliinase (Fig. 1) takes place extremely rapidly, a fact which is in agreement with the instantaneous appearance of the typical odor on crushing garlic. More than 80% of the alliin is split by the enzyme within 2 minutes (4). The molecular weight of the enzymes in onion and broccoli are quite similar (50, 64) and the enzyme appears to consist of a trimer with a subunit molecular weight of approximately 50,000. All of these enzymes are glycoproteins consisting of 5.8-6.0% carbohydrate by weight (50, 65). Cabbage leaves (66) and broccoli (50) have two cystine lyases with somewhat different specificities. Alliinase of Allium and cystine lyase of Brassica act on common substrate, S-alk(en)yl-Lcysteine sulfoxide (50) and Hamamoto & Mazelis (50) proposed a new name L-cysteine sulfoxide lyase. Optimum pH range of onion and broccoli enzymes is 8.0-8.6 (50, 69), and of garlic enzyme is 5-8 (4, 50). Action of alliinase on the mixture of sulfoxides forms allyl/methane, methyl/methane and other mixed thiosulfinates in addition to allicin (56). When studied with synthetic alliin, (-)-S-allyl-L-cysteine sulfoxide was disintegrated more slowly compared to its (+) isomer (54). In addition to methyl and propenyl derivatives of L-cysteine sulfoxide, as flavor precursors of onion, γ-L-glutamyl-L-cysteine sulfoxide is present and is thus insusceptable to the action of the C-S lyase (68). Since γ-L-glutamyl transpeptidase catalyzes the hydrolysis (Fig. 2) as well as glutamyl transfer, the addition of this enzyme to onion liberate the flavor precursor, which in turn destroyed by C-S lyase (67, 68). γ-L-Glutamyl transpeptidase is found in sprouted onion. In addition to already-described alliinase reaction common to Allium, alliinase of onion and leek, but not of garlic, has been reported to have an activity of generating lachrymatory compound, thiopropanal Soxide (70). Marks,et al. (17) reported the formation of MMTSO, methyl methanethiosulfonate (MMTSO2) and dimethyl trisulfide (DMTS) in a model system composed of SMCSO and partially purified cabbage C-S lyase. Bacteria are also known to have enzyme(s) that catalyzes the hydrolysis of S-alk(en)yl-L-cysteine sulfoxides. Stoll and Seebeck (4) reported the development of an odor of garlic, when E. coli was cultured in synthetic nutrient medium with 0.2% alliin. Bacterial enzymes of Pseudomonas cruciviae (71) and Bacillus subtilis (72) catalyzed the stoichiometric conversion of SMCSO to MMTSO, pyruvate and ammonia and required pyridoxal phosphate as a coenzyme (71), as other alliinases and C-S lyases did. SMCSO is converted to dimethyl disulfide (DMDS) by unspecified rumen microorganisms and cause hemolytic anaemia in cattle and sheep, known as kale poisoning (27). Figure 2. Liberation of L-cysteine sulfoxide from γ-glutamylpeptide in onion Products and Their Chemistry Cavallito and Bailey (3) succeeded in isolating a water-soluble antimicrobial substance from an aqueous ethanolic extract of garlic by steam distillation under reduced pressure and named it "allicin", and the Cavallito group (73) correctly assigned the structure as allyl-S(O)-S-allyl. Block et al. (10) made an HPLC analysis of thiosulfinates in garlic and reported that the major thiosulfinates from garlic and elephant garlic was allicin. Other kinds of thiosulfinates identified from the extracts of Allium species are as in Table 2. Sinha et al. (59) reported a finding of allicin from the supercritical CO 2 extract of onion. The discrepancy was explained by Block et al. (10) as an artifact due to very high injection port temperature employed by Sinha et al. (59). GC analysis employing high temperature may induce chemical modification of less stable compounds during the procedure of chromatography. Block (74) urges to use nonthermal methods of analysis like HPLC when volatile compounds of garlic are of interest. Allicin is not stable, even at 3℃, and it loses its activity within 14 days (4). Brodnitz et al. (75) observed that allicin underwent complete decomposition at 20℃ after 20hr resulting in DADS, diallyl trisulfide (DATS), diallyl sulfide and sulfur dioxide. But allicin in garlic juice underwent complete decomposition at 40℃ after 144hr (76). The authors postulated that allicin was more stable in garlic juice than in pure state. The particular instability of the allyl compound appears to be associated with the double bond (77). Thiosulfinates are unstable toward alkalies, but are stable in dilute acids. The generation of MMTSO was confirmed in a water extract of macerated Brussels sprouts which was the first evidence of MMTSO generated enzymatically under natural conditions (17). Unless stored at dry ice temperature, MMTSO, the primary breakdown product of SMCSO, has been shown to be degraded into volatile sulfur compounds, including methyl methanethiosulfonate (MMTSO2) and dimethyl disulfide (DMDS) (78, 79; Fig. 3). Figure 3. Spontaneous disproportionation of methyl methanethiosulfinate ANTIMICROBIAL COMPOUNDS DERIVED FROM S-ALK(EN)YL-L-CYSTEINE SULFOXIDES Before the nature of the antimicrobial substance of garlic is known as allicin by Cavallito and Bailey (3), some workers ascribed the garlic antimicrobial activity to other compounds including acrolein and related aldehydes (80). However, antimicrobial activity is not confined to allicin alone, but is a general property of alk(en)yl esters of alka(e)nethiosulfinate (4). The action of allicin, the first known natural thiosulfinate is considerably more bacteriostatic than bactericidal (3). It is about equally effective against Gram-positive and Gramnegative bacteria. A few observations were made by Small et al. (77) relative to the chemical structure and antimicrobial activity of the thiosulfinates. In general, it requires about the same quantities of the lower molecular weight thiosulfinates to inhibit Gram-positive as compared with Gram-negative bacteria, but as the carbon chain length increases, activity against Gram-negative organisms decreases, while that against Gram positive bacteria increases. Branching results in lowered activity. Small et al. (81) compared the antimicrobial activity of thiosulfinate and thiosulfonate (synthetic ethyl ethanethiosulfinate and ethyl ethanethiosulfonate, not found naturally). The two thiol esters were of comparable antimicrobial activity, with thiosulfonate being slightly more effective against S. aureus and Klebsiella pneumoniae. Figure 4. Formation of ajoene from allicin (Block et al., 1984) Ajoene, a derivative of allicin (Fig. 4), originally reported for its potent antithrombotic activity (25) exhibited a strong antifungal activity toward Aspergillus niger and C. albicans at <20μg/ml (82). Yoshida et al.(82) concluded that ajoene had stronger antifungal activity than allicin and that it damages the cell wall of fungi and thus maintained that growth inhibitory activity of ajoene toward bacteria was not expected except for a specific strain. Later Naganawa et al. (83) showed a different result concerning antimicrobial activity of ajoene. They reported that ajoene was strongly inhibitory against Gram-positive bacteria and yeasts and had various degrees of inhibition against Gram-negative bacteria like E. coli and P. aeruginosa. DADS, one of degradation products of allicin, was shown to possess antituberculosis activity (84). MMTSO, the primary breakdown product of SMCSO in Brassica (17, 48), has been shown to be antibacterial (48). MMTSO is not as potent as allicin. MMTSO decomposes on standing to give principally MMTSO2, DMDS and dimethyl trisulfide (DMTS) (17, 85), thus decreasing antimicrobial activity by approximately half, because MMTSO2 has comparable antimicrobial activity with MMTSO, but DMDS has only slight growth inhibitory activity. Kyung and Fleming (86) tested antimicrobial activity of SMCSO and its derivatives against 15 bacteria and 4 yeasts. SMCSO itself was uninhibitory while DMDS was only slightly inhibitory, and DMTS was weakly inhibitory. MMTSO2 was as inhibitory as MMTSO but with different inhibitory patterns. MMTSOO is also generated in autoclaved cabbage and SMCSO solution and was shown to be antibacterial (87). Cysteine inhibits antimicrobial activity of allicin, which may be reactivated by hydrogen peroxide (88). Antimicrobial activity of garlic was known to be stabilized by hydrogen peroxide (89). However, it is not in agreement with the report (24) that catalase positive bacteria were sensitive to garlic while less sensitive bacteria, e.g., lactic acid bacteria, were catalase negative. MODE OF ACTION The principal antimicrobial compounds of Allium and Brassica are those belonging to a group known as thiosulfinate. The antimicrobial activity of thiosulfinates has been explained as a general reaction between thiosulfinates and -SH groups of essential cellular proteins (41, 73, 77, 81). Small et al. (77) mentioned that S(O)S- was responsible for the antimicrobial activity and that reacted readily with cysteine to yield mixed disulfides. Fujiwara et al. (57) showed essentially the same reaction between allicin and thiamine. The general reaction (Fig. 5), as proposed by Small et al. (77) can apply to where thiosulfinates are involved and the reaction is believed to be the common mechanism of antimicrobial activity of thiosulfinates. Since the proposal of the general inhibitory mechanism of thiosulfinates, some workers (28-32) have reported specific target processes or enzymes of thiosulfinates. Ghannoum (30) and Neuwirth et al., (31) reported that allicin inhibited lipid biosynthesis and RNA synthesis, respectively, without pointing out target enzymes. Wills (28) reported that allicin inhibited the acticity of many -SH enzymes. Among them most strongly inhibited were xanthine oxidase, succinic dehydrogenase and triose phosphate dehydrogenase. He confirmed the results of Small et al. (77) that -S(O)-S- group was essential for the inhibition of -SH enzymes, while -S-S-, -S- and -SO- groups were not effective. Focke et al. (32) found that allicin inhibited the incorporation of acetate, but not of acetyl CoA or malonate, into fatty acids and concluded that only acetyl CoA synthetase for the fatty acid synthesis was inhibited by allicin. They explained that the inhibition of acetyl CoA synthetase by allicin was specific and non-sulfhydryl effect. Figure 5. Proposed reaction between thiosulfinates and SH group of cellular proteins Ajoene is reported to be another potent antimcirobial compound. Yoshida et al. (82), maintaining that ajoene was even more potent antifungal agent than allicin, assumed that ajoene may damage the cell walls of fungi, thus not expecting significant antibacterial activity, except for Staphylococcus aureus. Later, however, Naganawa et al. (83) showed a different result concerning antimicrobial activity of ajoene. They reported that ajoene was strongly inhibitory against Gram-positive bacteria and yeasts and had various degrees of inhibition of Gram-negative bacteria like E. coli and P. aeruginosa. Naganawa et al. (83) postulated that the disulfide group in ajoene appears to be necessary for the antibacterial activity, since reduction by cysteine abolished its antimicrobial activity. Ajoene does not possess thiosulfinyl group (Fig. 4). USES IN FOODS AS A PRESERVATIVE Although antimicrobial activity of Allium and Brassica vegetables represents a promising area of research, use of the vegetables as natural food preservatives has not been common. There is only one known example of using garlic as a food preservative. When Al-Delaimy and Barakat (90) treated ground camel meat with 5% or more of ground garlic, they could extend storage shelf-life of camel meat at any given storage temperature. Fifteen percent or more of garlic were found to act as strong bactericides since the initial microbial population of ground camel meat was completely destroyed and no further growth of any type of microorganisms was observed. Such a high level of garlic added to foods would be fine with some segments of population of the world, but not with the rest. A way to reduce the level of garlic added to foods for extending the shelf-life could be low temperature storage of them. For example, meat products with added garlic could be stored at the refrigerated temperature with extended shelf-life. However, there are difficult problems to be solved before garlic products are used as natural food additives, such as the strong garlic flavor and instability of functional compounds in garlic. CONCLUSION The antimicrobial activity of Allium and Brassica used as flavoring agents or food materials have been recognized for many years. Among the vegetables, garlic has been studied most extensively. The antimicrobial activity of Allium and Brassica is believed to be due to thiosulfinates enzymatically generated from S-alk(en)yl-L-cysteine sulfoxides. The antimicrobial action of thiosulfinates and their derivatives are effective due to -S(O)-S- group in the molecules, which readily react with -SH group of essential proteins of microorganisms. Ajoene and MMTSO2 which are generated from allicin and MMTSO, respectively, are also known to possess antimicrobial activity. Levels of garlic normally used as food-flavoring materials may not be sufficient to obtain the desired preservative effects. Garlic showed an acceptable preservative effect only when substantial levels were added to food, which may not be acceptable by many people because of the strong flavor. The relative instability of the activity further discourage the use of them as food preservatives. We have to acquire ways to enhance stability of antimicrobial compounds and to decrease objectionable sulfurous odor before finding more use of those vegetables possessing potent natural antimicrobial activities as a food preservative. REFERENCES 1 .Walton, L., Herbold, M. and Lindegren, C. C. (1936). Bactericidal effects of vapors from crushed garlic, Food Res. 1, 163-169. 2. Sherman, J. M. and Hodge, H. M. (1936). The bactericidal properties of certain plant juices, J. Bacteriol., 31, 96. 3. Cavallito, C. J. and Bailey, J. H. (1944). Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antimicrobial action, J. Amer. Chem. Soc., 66, 1950-1951. 4. Stoll, A. and Seebeck, E. (1951). Chemical investigation of alliin, the specific principle of garlic, Adv. Enzymol., 11, 377-400. 5. Kyung, K. H. and Fleming, H. P. (1994). S-Methyl-L-cysteine sulfoxide as the precursor of methyl methanethiosulfinate, the principal antibacterial compound in cabbage, J. Food Sci., 59(2), 350-355. 6. Virtanen, A.I. (1962). Some organic sulfur compounds in vegetables and fodder plants and their significance in human nutrition, Angew. Chem. (Int. ed.), 6, 299-306. 7. Zsolnai, V. T. (1966). Die antimicrobielle Wirkung von Thiocyanaten und Isothiocyanaten. I. Mitteilung, Arzneim. Forsch., 16, 870-876. 8. Delaquis, P. J. and Mazza, G. (1995). Antimicrobial properties of isothiocyanates in food preservation, Food Technol., 49(11), 73-84. 9. Virtanen, A. J. (1965). Studies on organosulfur compounds and other labile substances in plants, Phytochemistry, 4, 207-228. 10. Block, E., Naganathan, S., Putman, D. and Zhao, S.-H. (1992). Allium chemistry: HPLC analysis of thiosulfinates from onion, garlic, wild garlic (Ramsons), leek, scallion, shallot, elephant (great-headed) garlic, chive, and Chinese chive. Uniquely high allyl to methyl ratios in some garlic samples, J. Agric. Food Chem., 40, 2418-2430. 11. Pederson, C. S. and Fisher, P. (1944). The bactericidal action of cabbage and other vegetable juices, N.Y State Agric. Exp. Sta. Bull., 273. 12. Little, J. E. and Graubaugh, K. K. (1946). Antibiotic activity of some crude plant juices, J. Bacteriol., 52, 587-591. 13. Liu, J. Y., Teraoka, T., Hosokawa, D. and Watanabe, M. (1986). Bacterial multiplication and antibacterial activities in cabbage leaf tissue inoculated with pathogenic and nonpathogenic bacterium, Ann. Phytopath. Soc.(Japan) 52, 669-674. 14. Dickerman, J. M. and Lieberman, S. (1952). Studies on the chemical nature of an antibiotic present in water extract of cabbage, Food Res., 17, 438-441. 15. Synge, R. L. M. and Wood, J. C. (1956). (+)-(S-methyl-L-cysteine S-oxide) in cabbage, Biochem. J., 64, 252-259. 16. Morris, C. J. and Thompson, J. F. (1956). The identification of (+)S-methyl-L-cysteine sulfoxide in plants, J. Am. Chem. Soc., 78, 1605-1608. 17. Marks, H. S., Hilson, J. A., Leichtweis, H. C. and Stoewsand, G. S. (1992). S-Methylcysteine sulfoxide in Brassica vegetables and formation of methyl methanethiosulfinate from Brussels sprout, J. Agric. Food Chem., 40, 2098-2101. 18. Marks, H. S. (1992). Isolation, identification, and genotoxic inhibitory efficacy of naturally occurring organosulfur compounds present in Brassica vegetables, Cornell Univ., Doctoral Diss. 19. Marks, H. S., Leichtweis, H. C. and Stoesand, G. S. (1991). Analysis of a reported organosulfur, carcinogen inhibitor; 1,2-dithiole-3-thione in cabagge, J. Agric. Food Chem., 39, 893-895. 20. Caragay, A. B. (1992). Cancer-preventive foods and ingredients, Food Technol., 46, 65-68. 21. Alberto-Puleo, M. (1983). Physiological effects of cabbage with reference to its potential as a dietary cancer-inhibitor and its use in ancient medicine, J. Ethnopharmacol., 9, 261-272. 22. Cheney, G. (1950). Anti-peptic ulcer dietary factor (vitamin U) in the treatment of peptic ulcer, J. Am. Dietet. Ass., 26, 668-672. 23. Augusti, K. T. and Mathew, P. T. (1974). Lipid lowering effect of allicin (diallyl disulphide-oxide) on long term feeding to normal rats, Experientia, 30(5), 468-470. 24. Rees, L. P., Minney, S. F., Plummer, N. T., Slator, J. H. and Skyrme, D. A. (1993). A quantitative assessment of the antimicrobial activity of garlic (Allium sativum), World J. Microbiol. Biotechnol., 9, 303-307. 25. Block, E., Ahmed, S., Jain, M.K., Creely, R. W., Apitz-Castro, R. and Cruz, M. R. (1984). (E,Z)-Ajoene: A potent antithrombotic agent from garlic, J. Am. Chem. Soc., 106, 8295-8296. 26. Whittle, P. J., Smith, R. H. and McIntosh, A. (1976). Estimation of S-methylcysteine sulphoxide (kale anaemia factor) and its distribution among Brassica forage and root crops, J. Sci. Food Agric., 27, 633-642. 27. Smith, R. H. (1980). Kale poisoning: The brassica anaemia factor, Veter. Record, 107, 12-15. 28. Wills, E. D. (1956). Enzyme inhibition by allicin, the active principle of garlic, Biochem. J., 63, 514-520. 29. Adetumbi, M., Javor, G. T. and Lau, B.H.S. (1986). Allium sativum (garlic) inhibits lipid synthesis in Candida albicans, Antimicrob. Agents Chemother., 30, 499-501. 30. Ghannoum, M. A. (1988). Studies of the antimicrobial mode of action of Allium sativum (garlic)J. Gen. Microbiol., 134, 2917-2924. 31. Feldberg, R. S., Chang, S. C., Kotik, A.N., Nadler, M., Neuwirth, Z., Sundstrom, D. C. and Thompson, N. H. (1988). In vitro mechanism of inhibition of bacterial cell growth by allicin, Antimicrob. Agents Chemother. 32, 1763-1768. 32. Focke, M., Feld, A. and Lichtenthaler, H. K. (1990). Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase, FEBS, 261(1), 106-108. 33. Dababneh, . F. A. and Al-Delaimy, K. S. (1984). Inhibition of Staphylococcus aureus by garlic extract, Lebensm. Wissenschaft. Technol., 17(1), 29-31. 34. Karaioannoglou, P. G., Mantis, A. J. and Panetos, A. G. (1977). The effects of garlic extract on lactic acid bacteria (Lactobacillus plantarum) in culture media, Lebensm. Wissenschaft. Technol., 10, 148-150. 35. Mantis, A. J., Karaioannoglou, P. G., Spanos, G. P. and Panetos, A. G. (1978). The effect of garlic extract on food poisoning bacteria in culture media. I. Staphylococcus aureus, Lebensm. Wissenschaft. Technol., 11, 26-28. 36. Conner, D. E. and Beuchat, L. R. (1984). Effects of essential oils from plants on growth of food spoilage yeasts, J. Food Sci., 49, 429-434. 37. Johnson, M. G. and Vaughn, R. H. (1969). Death of Salmonella typhimurium and Escherichia coli in the presence of freshly reconstituted dehydrated garlic and onion, Appl. Microbiol., 17(6), 903-905. 38. Saleem, Z. M. and Al-Delaimy, K. S. (1982). Inhibition of Bacillus cereus by garlic extracts, J. Food Prot., 45(11), 1007-1009. 39. DeWit, J. C., Notermans, S., Gorin, N. and Kampelmacher, E. H. (1979). Effects of garlic oil or onion oil on toxin production by Clostridium botulinum in meat slurry, J. Food Prot., 42, 222-224. 40. Mantis, A. J., Koidis, P. A., Karaioannoglou, P. G. and Panetos, A. G. (1979). Effect of garlic extract on food poisoning bacteria, Lebensm. Wissenschaft. Technol., 12, 230-232. 41. Barone, F. E. and Tansey, M. R. (1977). Isolation, purification, identification, synthesis, and kinetics of activity of the anticandidal component of Allium sativum, and a hypothesis for its mode of action, Mycologia, 69, 793-825. 42. Al-Delaimy, K. S. and Ali, S. H. (1970). Antibacterial action of vegetable extracts on the growth of pathogenic bacteria, J. Sci. Food Agric., 21, 110-112 43. Srivastava, K. C., Perera, A. D. and Saridakis, H. O. (1982). Bacteriostatic effects of garlic sap on Gram negative pathogenic bacteria - an in vitro study, Lebensm. Wissenschaft. Technol., 15(2), 74-76. 44. Moore, G. S. and Atkins, R. D. (1977). The fungicidal and fungistatic effects of an aqueous garlic extract on medically important yeast-like fungi, Mycologia, 69, 341-348. 45. Tansey, M. R. and Appleton, J. A. (1975). Inhibition of fungal growth by garlic extract, Mycologia, 67, 409-413. 46. Singh, K. V. and Shukula, N. P. (1984). Activity of multiple resistant bacteria of garlic (Allium sativum) extract, Fitoterapia, 15(5), 313-315. 47. Shin, D.B., Kim, Y.S. and Lee, Y.C. (1999). Effect of dehydration methods on the antimicrobial activity of garlic juice powder, 1999 IFT Annual Meeting, 98. 48. Kyung, K. H. and Fleming, H. P. (1994). Antibacterial activity of cabbage juice against lactic acid bacteria, J. Food Sci., 59(1), 125-129. 49. Yildiz, F. and Westhoff, D. (1981). Associative growth of lactic acid bacteria in cabbage juice, J. Food Sci., 46, 962-963. 50. Hamamoto, A. and Mazelis, M. (1986). The C-S lyases of higher plants. Isolation and properties of homogeneous cystine lyase from broccoli, Plant Physiol., 80, 702-706. 51. Mae, T., Ohira, K. and Fujiwara, A. (1971). Fate of (+)S-methyl-L-cysteine sulfoxide in Chinese cabbage, Brassica pekinensis RUPR, Plant Cell Physiol., 12, 1-11. 52. Rundqvist, C. (1909). Pharmacological incestigation of Allium bulbs, Pharmaceutiskt Notisblad, 18, 323333. 53. Stoll, A. and Seebeck, E. (1948). Uber Alliin, die genuine Muttersubstanz des Knoblauchols. 1. Mitteilung uber Allium Substanzen, Helv. Chim Acta, 31, 189-210. 54. Stoll, A. and Seebeck, E. (1951). Die Synthese des naturlichen Alliins und seiner drei optisch aktiven Isomeren. 5. Mitteilung uber Allium-Substanzen, Helv. Chim. Acta, 34, 481-487. 55. Ziegler, S. J. and Sticher, O. (1989). HPLC of S-alk(en)yl-L-cysteine derivatives in garlic including quantitative determination of (+)S-allyl-L-cysteine sulfoxide (alliin), Planta Medica, 55, 372-377. 56. Freeman, G. G. and Whenham, R. J. (1975). The use of synthetic (+_)-S-1-propyl-L-cysteine sulphoxide and of alliinase preparations in studies of flavour changes resulting from processing of onion ( Allium cepa L.), J. Sci. Food Agric., 26, 1333-1346. 57. Fujiwara, M., Yoshimura, M., Tsuno, S. and Murakami, F. (1958). Allithiamine, a newly found derivative of vitamin B1. IV. On the allicin homologues in the vegetables, J. Biochem. (Japan), 45, 141-149. 58. Horhammer, L., Wagner, H., Seitz, M. and Vejdelek, Z. J. (1968). Onion flavors and their analysis by gas chromatography-mass spectrometry, Pharmazie, 23, 462-466. 59. Sinha, N. K., Guyer, D. E., Gage, D. A. and Lira, C. T. (1992). Supercritical carbon dioxide extraction of onion flavors and their analysis by gas chromatography-mass spectrometry, J. Agric. Food Chem., 40, 842845. 60. Kunelius, H. T., Sanderson, J. B. and Narashimhalu, P. R. (1987). Effect of seeding date on yields and quality of green forage crops, Can. J. Plant Sci., 67, 1045-1050. 61. Bradshaw, J. E. and Borzucki, R. (1982). Digestability, S-methyl-L-cysteine sulfoxide content and thiocyante ion content of cabbage of stockfeeding, J. Sci. Food Agric., 33, 1-5. 62. Arnold, W. N. and Thompson, J. F. (1962). The formation of (+)S-methyl-L-cysteine sulfoxide from Smethyl-L-cysteine in crucifers, Biochim. Biophys. Acta, 57, 604-606. 63. Lancaster, J. E. and Collin, H. A. (1981). Presence of alliinase in isolated vacuoles and of alkyl cysteine sulfoxides in cytoplasm of bulbs of onion (Allium cepa), Plant Sci. Lett., 22, 169-176. 64. Anderson, N. W. and Thompson, J. F. (1979). Cystine lyase: beta-cystathionase from turnip roots, Phytochemistry, 18, 1953-1958. 65. Nock, L. P. and Mazelis, M. (1985). Some new observations on the properties of garlic alliinase, Plant Physiol., 77, S-115 66. Hall, D. I. and Smith, I. K. (1983). Partial purification and characterization of cystine lyase from cabbage (Brassica oleraceae var capitata), Plant Physiol., 72, 654-658. 67. Schwimmer, S. (1971). Enzymatic conversion of gamma-L-glutamyl cysteine peptides to pyruvic acid, a coupled reaction for enhancement of onion flavor, J. Agric. Food Chem., 19, 980-983. 68. Schwimmer, S. and Austin, S. J. (1971). Enhancement of pyruvic acid release and flavor in dehydrated Allium powders by gamma glutamyl transpeptidases, J. Food Sci., 36, 1081-1085. 69. Mazelis, M. (1963). Demonstration and characterization of cysteine sulfoxide lyase in cruciferae, Phytochemistry, 2, 15-22. 70. Freeman, G. G. (1975). Distribution of flavor components in onion (Allium cepa L.), leek (Allium porrum) and garlic (Allium sativum), J. Sci. Food Agric., 26, 471-481. 71. Nomura, J., Nishizuka, Y. and Hayaishi, O. (1963). S-Alkylcysteinase : Enzymatic cleavage of S-methylL-cysteine and its sulfoxide, J. Biol. Chem., 238(4), 1441- 1446. 72. Murakami, F. (1960). Studies on the nutritional value of Allium plants (XXXVI) Decomposition of alliin homologues by microorganism and formation of substance with thiamine masking activity, Vitamins (Tokyo), 20, 126. 73. Cavallito, C. J., Buck, J. S. and Suter, C. M. (1944). Allicin, the antibacterial principles of Allium sativim. II. Determination of the chemical structure, J. Am. Chem. Soc., 66, 1952-1954. 74. Block, E. (1993). Flavor artifacts, J. Agric. Food Chem., 41, 692 75. Brodnitz, M. H., Pascale, J. V. and von Derslice, L. (1971). Flavor components of garlic extract, J. Agric. Food Chem., 19, 273-275. 76. Yu, T.-H. and Wu, C.-M. (1989). Stability of allicin in garlic juice, J. Food Sci., 54(4), 977-981. 77. Small, L. D., Bailey, J. H. and Cavallito, C. J. (1947). Alkyl thiosulfinates, J. Am. Chem. Soc., 69, 17101713. 78. Moore, T. L. and O'connor, D. E. (1966). The reaction of methanesulfenyl chloride with alkoxides and alcohols. Preparation of aliphatic sulfenate and sulfinate esters, J. Org. Chem., 31, 3587-3592. 79. Ostermayer, F. and Tarbell, D. S. (1960). Products of acidic hydrolysis of S-methyl-L-cysteine sulfoxide; isolation of methyl methanethiosulfinate and mechanism of the hydrolysis, J. Am. Chem. Soc., 82, 3752-3755. 80. Ingersoll, R. L., Volirath, R. E., Scott, B. and Lindegren, C. C. (1938). Bactericidal activity of crotonaldehyde, Food Res., 3, 389-392. 81. Small, L. D., Bailey, J. H. and Cavallito, C. J. (1949) Comparison of some properties of thiosulfonates and thiosulfinates, J. Am. Chem. Soc., 71, 3565-3566. 82. Yoshida, S., Kasuga, S., Hayashi, N., Ushiroguchi, T., Matsuura, H. and Nakagawa, S. (1987). Antifungal activity of ajoene derived from garlic, Appl. Environ. Microbiol., 53(3), 615-617. 83. Naganawa, R., Iwata, N., Ishikawa, K., Fukyda, H., Fujino, T. and Suzuki, A. (1996). Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic, Appl. Environ. Microbiol., 62(11), 42384242. 84. Brown, H. D., Matzuk, A. R., Becker, H. J., Conbere, J. P., Constantin, J. M., Solotorovsky, M., Winsten, S., Ironson, E. and Quastel, J. H. (1954). The antituberculosis activity of some ethylmercapto compounds, J. Am. Chem. Soc., 76, 3860 85. Chin, H.-W. and Linsay, R. C. (1994). Mechanisms of formation of volatile sulfur compounds following the action of cysteine sulfoxide lyases, J. Agric. Food Chem., 42, 1529-1536. 86. Kyung, K. H. and Fleming, H. P. (1997). Antimicrobial activity of sulfur compounds derived from cabbage, J. Food Prot., 60(1), 67-71. 87. Kyung, K. H., Han, D. C. and Fleming, H. P. (1997). Antimicrobial activity of heated cabbage juice, Smethyl-L-cysteine sulfoxide and methyl methanethiosulfonate, J. Food Sci., 62(2), 406-409. 88. Kubelik, J. (1970). Antimicrobielle Eigenschaften des Knoblauchs, Pharmazie, 25, 266-269. 89. Lubeck, V. L. (1956). Verfahren zum Stabilisieren von antibiotisch wirksamen Inhaltsstoffen von Allium sativum, Deutch. Pat. 943250. 90. Al-Delaimy, K. S. and Barakat, M. M. F. (1971). Antimicrobial and preservative activity of garlic on fresh ground camel meat. I. Effect of fresh ground garlic segments, J. Sci. Food Agric., 22, 96-98. Legends for Tables Table 1. Kinds and amounts of S-alk(en)yl-L-cysteine sulfoxides in Allium and Brassica Table 2. Thiosulfinates from the extracts of Allium and Brassica Legends for Figures Figure 1. Enzymatic cleavage of S-alk(en)yl-L-cysteine sulfoxides Figure 2. Liberation of L-cysteine sulfoxide from gamma-glutamylpeptide in onion Figure 3. Spontaneous disproportionation of methyl methanethiosulfinate Figure 4. Formation of ajoene from allicin Figure 5. Proposed reaction between thiosulfinates and SH group of cellular proteins