Chemistry
advertisement

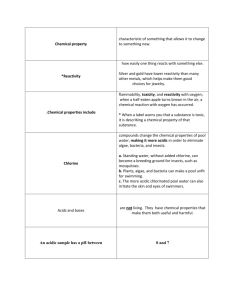
Cardiff International School Dhaka (CISD) Lost Class Make Up Assignment Class: 10A Subject: Chemistry th Date: 8 (Sunday)-14th (Saturday) February 2015 Total Mark- 20 Instructions: All of your assignment must be done in A4 size paper. Mention your Name, Class, Roll and Section clearly on the top sheet of your assignment. Submission Deadline: Saturday 14th February 10.00 AM to the respective subject teacher. The deadline is strict. Name:......................................................................................................................................................... Class: ......................... Roll: ........................ Sec: .................. Teacher: ............................................. Sunday-Saturday 08-14 February 2015 Lesson: 1) Define acids, bases & alkali 2) Difference between a. strong and weak acids, b. strong and weak bases 3) Properties of acids i. Reaction of acids with metal ii. Reaction of acids with metal oxide iii. Reaction of acids with metal hydroxide iv. Reaction of acids with metal carbonates 4) Properties of bases i. Reaction of bases with acids ii. Reaction of bases with ammonium salts 5) Define Neutralization reaction and writing ionic equation 6) How the pH value changes during neutralization reaction 7) Define indicator. Describing the color change of different indicators in acids, bases and neutral solution. Asa. Litmus solution/paper, b. methyl orange, c. phenolphathellin and d. universal indicator H 8) p scale and its value for strong and weak acids-bases and neutral solution 9) What substances are added into soil to control the acidity of soil and why it is important to control soil acidity? 10) Define salt and how it is formed. Example of soluble and insoluble salt. 11) Methods of preparing soluble and insoluble salts and How to recover the salt from its solution Task: Read the lessons carefully mentioned above and answer the following questions. Worksheet/Exercise: Q1 Ethanoic acid is a weak acid. It reacts with magnesium giving a gas and a magnesium salt. (i) What is meant by the term weak acid? (ii) Name the gas formed. (iii) What is the formula of the magnesium salt formed? [4] Q 2.Fertilisers are soluble salts containing one or more of the essential elements required for plant growth. Ammonium chloride can be prepared by the reaction between aqueous ammonia and hydrochloric acid. Write an ionic equation for this reaction. [3] Q3. A student places a rod of magnesium in aqueous silver nitrate. (i) Write an ionic equation, with state symbols, for the reaction which happened. (ii) What would you expect to see after the reaction had been taking place for some time? [4] Q4. This question is about making salts. For each salt, suggest the name of the missing reagent and briefly describe how to obtain the solid product from the reaction mixture. (i) Salt to be made: lithium chloride. reagent 1: dilute hydrochloric acid reagent 2: ............................... I could obtain solid lithium chloride by: ……………………… (ii) Salt to be made: barium sulphate. reagent 1: aqueous potassium sulphate reagent 2: ............................................. I could obtain solid barium sulphate by: ........................................... (iii) Salt to be made: blue copper(II) sulphate crystals. reagent 1: dilute sulphuric acid reagent 2: ...................................................................... I could obtain blue copper(II) sulphate crystals by:………………… Answer Key: 1) i. Partially ionized ii. Hydrogen gas iii. Magnesium ethanoate 2) H+ + OH- H2O 3) i. Mg + 2Ag+ Mg+ + 2Ag ii. Magnesium rod decreases in size, silvery solid deposits 4) i. Lithium hydroxide, titration ii. Barium nitrate, filtration iii. Copper oxide, crystalization [9] Text Book/Reference Book: Chemistry Matters, Chapter-11, 12, pg- 169- 203 Help Lines: For any assistance, please contact 1. Chemistry Teacher: Shafiqul Islam Patuwary, +8801937075363 2. Principal Head of School: G.M.Nizam Uddin, +88-01622181818, gmnu302@yahoo.com