Acids and bases answers
advertisement

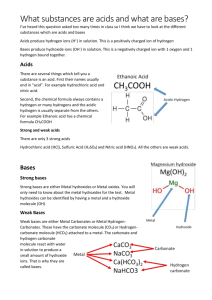
Acids and bases - Answers Revision questions 1. Acids are substances that are sour and have a pH value of less than 7. Bases are substances that are bitter and have a pH value of greater than 7. 2. Acids are sour, corrosive and have a pH value of less than 7. Bases are bitter, corrosive and have a pH value of greater than 7. 3. Indicators are to test substances to see whether they are acids or bases. 4. Alkaline 5. (i) HCl (ii) H2SO4 (iii) NH3 6. (i) Acid + metal → water + salt (ii) Acid + carbonate → water + salt + carbon dioxide (iii) Nitric acid + magnesium → hydrogen + magnesium nitrate (iv) Sulfuric acid + calcium hydroxide → water + calcium sulfate (v) Hydrochloric acid + aluminium carbonate → water + carbon dioxide + aluminium chloride 7. A metal reacts with oxygen to form a metal oxide. This is known as rust. 8. Magnesium + oxygen → magnesium oxide Past SC questions on acids and bases 2010 1) Multiple choice (i) D 2) One world answers (i) Indicators 3) Short answer questions (i) Wear safety glasses to prevent acids from accidentally splashing into your eyes. Acids are corrosive and so can damage your eyes. (ii) Temperature change; the magnesium dissolving into the hydrochloric acid (iii) Carbon dioxide 2009 1) Multiple choice (i) A (ii) A 2) One word answers (i) Hydrogen (ii) Indicators 2008 1) Multiple choice (i) C (ii) A (iii) A 2006 1) Multiple choice (i) B 2005 1) Multiple choice (i) D (ii) A (iii) B 2) One word answers (i) Sulfuric acid 2004 1) Multiple choice (i) A (ii) B (iii) D 2) One word answers (i) Corrosion 3) (a) (i) Metal (b) (i) Acid can splash on your eyes (ii) Safety glasses can be worn to prevent acids from getting into your eyes (ii) Temperature change, gas produced and metal dissolving into the acid