Physical-chemical and biological measures of water quality are the

YEAR 12 SCIENCE21
WATER QUALITY TESTING PROCEDURE
BOOKLET
1.
Temperature
2.
pH
3.
Turbidity
4.
Total Solids
5.
Dissolved Oxygen (D.O.)
6.
Biochemical Demand (B.O.D.)
7.
Total Phosphorus (phosphates)
8.
Nitrates
9.
Faecal Coliform (F.C.)
10.
Critter Countdown (used as evidence only NOT for WQI)
11.
Chlorine/Salinity (used as evidence only NOT for WQI)
HOW TO MEASURE WATER QUALITY
It is necessary to learn how relatively untouched; natural river ecosystems work in order to assess the magnitude of changes occurring in water quality.
It is also important to recognize our involvement in water quality problems to enable us to amend any actions that degrade the river ecosystem. These may include the local factors within the catchment which may influence the water quality of the river.
Many factors can affect the water quality of a river system. The conditions of a river can fluctuate periodically; therefore, we must measure water quality periodically. Water that is determined safe for one use may be unacceptable for another purpose. In fact, many water quality experts refer to their measurements in terms of a specific use. In Australia the four main use categories are: drinking, irrigation, recreational and environmental.
In an attempt to devise a system to compare rivers in various parts of the country, the
American National Sanitation Foundation (NSF) has created and designed a standard index, called the Water Quality Index (WQI). The NSFWQI is one of the most widely used of all existing water quality indices. It was developed in 1970, and can reach over time, compare water quality from different reaches of the same river, and even compare water quality of different rivers.
The results can also be used to determine if a particular use of the water is healthy.
•
•
•
•
THE 9 TESTS
To determine the WQI, nine tests must be performed. These are:
• Dissolved oxygen
Faecal coliform pH
Biochemical oxygen demand (5-day)
Temperature
•
•
•
•
Total phosphorus
Nitrates
Turbidity
Total solids
•
•
Other tests:
Critter count
Chlorine/Salinity
WHAT IS THE WQI & WHAT DOES IT MEAN?
After completing the nine tests, the results are recorded and transferred to a weighing curve chart where a numerical value is obtained. The numerical value or Q-value for each test or parameter is multiplied by a “weighing factor” for each parameter. Dissolved oxygen has a relatively high weighting factor (0.17) and therefore is more significant in determining water quality than the other parameters. The nine values are added to derive an overall water quality index (WQI).
90 – 100
70 – 90
50 – 70
Excellent
Good
Medium
25 – 50
0 – 25
Bad
Very Bad
It has been determined that a minimum WQI rating of 50-58 would be required to meet most water quality standards. However, it is important to note that a body of water could have a WQI above this range and still fail to meet state standards. For example, if the water quality index falls within the “good” range (70-90) and the faecal coliform bacterial level exceeds 200 colonies/100ml, then this water does not meet state standard for total body contact like swimming. As you run the water quality tests, use the weighted curve charts, and derive a water quality index, this entire process will become clear.
SAMPLING PROCEDURES WHEN MEASURING WATER QUALITY
It is important to exercise care in the way samples are collected for analysis. A collected sample should be representative of the river reach being tested. Analytical values derived from river samples may vary with depth, velocity of current, and distance the sample was taken from shore.
With these thoughts in mind, sampling from shore is not the preferred method of sampling. Near-shore samples are usually not representative of the river at that location. If possible, water samples should be collected from a bridge spanning the river, from a boat, or off the end of a dock. A rule of thumb for sampling is to sample midway across the river and midway between the surface and bottom. A simple sampling device can be constructed from a series of metal rods that can be extended and rubber tubing attached that holds the sample bottle, (see figure 5). This device might be extended out from shore if no bridges are available and particularly if the river is narrow and shallow.
The samples most often taken by students are called “grab samples”, which are single samples representative of the river at a particular time and place. To improve the reliability of the water quality data collected; replicate samples (additional samples) should be taken from a particular location. For example, more than one group could run a dissolved oxygen test at the same river location.
A mixture of grab samples collected at the same sampling point at different times is called a “composite sample”. If a number of classes collect grab samples over the course of a day at the same sampling location then this would also be a form of composite sampling but only of an average value is calculated from all the samples. For example, if four different classes each ran a nitrate test at the same sampling point then combined these individual nitrate values to compute an average value, and then this would be a form of composite sampling.
It is important to remember that the shorter the time between sampling and analysis, the more reliable the results will be. The dissolved oxygen, temperature, and pH tests should be completed at the time of sampling as results for these tests change with time.
If the purpose of sampling is to note change in Water Quality Index from year to year, then it is important to run the water quality tests at approximately the same ween and time of the day each year. Before sampling it is advisable to carefully review the sampling procedures and water quality testing directions for the particular water quality test(s) to be done.
TEMPERATURE
Temperature Background Information
Many of the physical, biological, and chemical characteristics of a river are dependent upon temperature.
Temperature affects the following characteristics:
The solubility of oxygen in water
The rate of photosynthesis by algae and larger aquatic plants
The metabolic rates of aquatic organisms (For every 10 o rise in temp the metabolic rate doubles)
The sensitivity of organisms to toxic wastes, parasites, and diseases.
Amplification of tastes and odours
Gases are more soluble in cool water than in warm water, therefore cool water can hold more oxygen than warm water. As water temperature increases, increased growth of certain algae and plankton (microscopic floating plants and animals) can occur. An increase in plant growth and photosynthesis means more oxygen is produced, but it also means more plant respiration and decay will use oxygen.
The metabolic rate of organisms also increases with increasing water temperature. Increased metabolism increases the oxygen demand of organisms like fish, aquatic insects, and aerobic bacteria. Very high or low water temperatures may exceed the tolerance limit for aquatic organisms. Some aquatic organisms like trout and stonefly nymphs require cooler water temperatures than carp and dragonfly nymphs. Increasing water temperatures also accelerates the life cycles of many aquatic insects. This may lead to undesirable consequences for animals, which feed, on these insects, particularly birds that depend on emerging insects during their migration flight.
Many fish species become more vulnerable to damage from toxic wastes, parasites, and diseases with increasing temperatures. Fish become more vulnerable because they are in a weakened condition from lack of dissolved oxygen, or under stress from higher water temperatures.
What are some human influences or impacts upon river temperature?
During the hot summer months, the sun heats up urban surfaces such as streets, sidewalks, and parking lots.
Storm water runoff from these hot surfaces contributes warmer water to the river. Another source of warmer water comes from cooling water of industry and other industrial wastes. A good example if cooling water discharge is from sugar mills; this water is usually warmer than receiving waters and is called thermal pollution.
People may impact river temperatures by cutting down trees, which help shade the river from direct sunlight.
People may also contribute to warmer water by causing soil erosion along the riverbank. Erosion can be caused by removing trees and other vegetation, poor farming practices (ploughing near the bank), highway construction, and other construction. Soil erosion increases the amount of suspended solids carried by the river. Suspended solids make the water turbid or very cloudy. This cloudy water absorbs the sun’s rays, warming the water.
Temperature
Greater than 20
Upper range
(13 - 20 C)
Less than
20 C
(Cold Water)
Lower range
(Less than 13 C)
C.
Examples of Life
Much plant life, many fish (warm water) diseases.
Most bass, barramundi, carp, red claw and catfish
Some plant life, some fish diseases. Salmon, trout, red claw.
Stonefly nymphs, mayfly nymphs, caddisfly larvae, water beetles, and water striders.
Trout, caddisfly larvae, stonefly nymphs, and mayfly nymphs
Temperature tolerance levels of selected aquatic organisms.
The test for temperature, however, is not a measure of the water temperature in centigrade or Fahrenheit. It is a measure of the change in temperature between two points—the test site and one mile upstream. By discovering reaches of the river which undergo rapid temperature changes, we can learn if the river system is stressed from outside sources.
Expected Levels
Water temperatures can range from 0°C in the winter to above 30°C in the summer. Cooler water in a stream is generally considered healthier than warmer water, but there are no definitive standards. Problems generally occur when changes in water temperature are noted along one stream on the same day.
Summary of Method
You will use a Vernier Temperature Probe to measure the temperature of the water at one site and at a second site upstream (one mile upstream is the standard, but a shorter distance may be used). Because water temperatures can fluctuate so greatly from region to region and season to season, it is the difference in temperatures along one stretch of a particular stream or river on a single day that is measured.
Procedure – Temperature
1.
Place the tip of the probe into the stream at Site 1, or into a cup with sample water just taken from the stream.
2.
Submerge the probe tip to a depth of about 6 cm.
3.
Important: Leave the probe tip submerged for the 10 seconds that data is being collected.
4.
When the sampling run is complete, record the average temperature
Return to Step 1 to obtain a second reading
pH
pH Background information
Water (H
2
0) contains both H + (hydrogen) ions and OH (hydroxide) ions. Ions are electrically charged atoms or groups of atoms. The pH measures a quantity that is related to the hydrogen ion concentration. This measurement tells us if a solution is acidic or a basic. pH values usually lie between 1 and 14. Pure deionized water contains an equal number of hydrogen (H + ) and hydroxide (OH neutral, neither acidic nor basic. If a solution has more H + than OH -
) ions and has a pH of 7. It is considered
ions, it is ACIDIC and has a pH value of
LESS THAN SEVEN. If there are less H + than OH ions in the solution, it is a BASIC and has a pH value of
MORE THAN SEVEN. Each number on the scale represents a ten-fold change in the concentration of ions. For example, an acid having a pH of 4 is 100 times more powerful than an acid with a pH of 6.
In Australia, the pH of natural freshwaters is usually between 6.5 and 8.5, although wide variations can occur.
The natural decomposition of organic matter (dead animals, leaves etc) by bacteria, protozoa and fungi, produces organic acids that decrease the pH of the water. Various rocks and minerals present in the surrounding soil may also naturally influence the pH of the water. If limestone is present, the alkaline (basic) limestone neutralizes the effect acids might have on creeks and streams.
Changes in the pH value of water affect the organisms that live there. Most aquatic organisms have adapted to a specific pH and may die if even slight pH changes occur. Immature stages of aquatic insects and young fish are extremely sensitive to low pH values. Abnormally low pH values are usually found downstream of urban/industrial areas. When acid waters come into contact with certain chemicals and metals that occur in soils and bedrock, they often release them into the waters making them more toxic (poisonous) than normal.
Very acidic waters (<4.5) can also cause heavy metals, such as copper and aluminium, to be released into the water. Heavy metals can collect on fish gills or cause deformities in young fish, making it harder for them to survive. An abnormally high pH (>9.0) results in the dysfunction of the gills in many aquatic animals.
pH ranges that support aquatic life.
MOST ACIDIC NEUTRAL MOST ALKALINE
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Bacteria 1.0 13.0
Plants (algae, rooted, etc.) 6.5 12
Carp, suckers, catfish, some insects 6.0 9.0
Bass, bluegill, crappie 6.5 8.5
Snails, clams, mussels 7.0 9.0
Largest variety of animals 6.5 7.5
(trout, mayfly nymphs, caddisfly larvae)
What causes changes in pH?
A very important characteristic of a water sample is its ability to resist change in pH. This characteristic occurs when buffers are present in the water. The extent to which pH change can be resisted is called the buffering capacity of the solution. Water changes pH more readily if it is poorly buffered. Highly buffered water does not readily change pH. Distilled and rain water are poorly buffered solutions and will change pH readily while hard waters such as bore water (containing a lot of calcium or iron) and sea water are considered highly buffered solutions and don’t readily change pH. To put this in perspective, if a sample of rain water and sea water were taken and the scientist wished to adjust the pH of both samples to 8 (slightly basic), one drop of 0.1M sodium hydroxide may be needed for adjustment of the rain water sample while 20ml of 0.1M sodium hydroxide may be needed for the adjustment of the sea water. This property of buffering has lead to solutions of known pH to be “buffered” using high concentrations of phosphate to make the pH value extremely stable and these solutions can then be used to calibrate the pH meter. That is why the calibration solutions, usually the pink pH4 and the green pH 7, are called buffer solutions.
The presence of nitrogen oxides and sulphur dioxide can cause the water to become acidic. This is often found to be a problem in landfill sites in wet climates. As water leaches through rubbish it reacts with the nitrogen oxides and sulphur dioxide to form an acidic leachate. Even carbon dioxide can make poorly buffered waters acidic. For example rain water is poorly buffered and as it passes through the atmosphere it comes into contact with carbon dioxide (CO
2
) to form a weak carbonic acid, thus making rainwater slightly acidic (5.5-6pH)
Water may also become acidic through the process known as acid mine drainage. During the mining process a substance known as pyrite (FeS
2
) is freshly exposed to the surface. The pyrite oxidises i.e. reacts with water and oxygen to form sulphuric acid, which is then leached through the surrounding environment. Acid mine drainage can be problematic in dry areas of the country as these ecosystems are very fragile and can’t dilute pollutants as quickly as areas with higher water flow.
Another process that forms acidic water is the disturbance of acid sulphate soils. Acid sulphate soils were formed 10 000 years ago when sea levels were much higher, thus acid sulphate soils are mostly found in swampy coastal areas such as the mangrove swamps of Cairns. These soils contain, as the name suggests, sulphides, usually in the form of pyrite and are harmless when left undisturbed. As with acid mine drainage, these soils oxidise with air to form sulphuric acid when disturbed and brought to the surface, usually by excavation and clearing of mangroves for development. An example of such an area is East Trinity in Cairns. Acid sulphate soils can be problematic as they often occur in mangrove and estuarine areas, which are primary, fish hatchery and nursery habitats.
Expected Levels
The pH value of streams and lakes is usually between pH 7 and 8. Levels between 6.5 and 8.5 pH are acceptable for most drinking water standards. Areas with higher levels of water hardness (high concentrations of Mg2+,
Ca2+, and HCO3 –) often have water with higher pH values (between 7.5 and 8.5).
Summary of Methods
The preferred method is to use a pH Sensor to make on-site measurements of the pH level in a stream or lake.
As an alternative, the water sample is taken from the stream or lake and stored in an ice chest or refrigerator.
After returning to the lab, samples are allowed to return to room temperature, and the pH is measured using a pH Sensor.
Method – pH
1.
Remove the pH Sensor from the storage bottle. Rinse the tip of the sensor thoroughly with stream water.
2.
Place the tip of the probe into the stream at Site 1 or into a cup with sample water just taken from the stream. Submerge the sensor tip to a depth of 3–4 cm.
3. Important: Leave the probe tip submerged for the 10 seconds that data is being collected.
4. When the sampling run is complete, record the average pH.
5. Return to Step 1 to obtain a second reading
TURBIDITY
Turbidity Background Information
Turbidity is the result of suspended solids in the water. Turbidity is the opposite of clarity. Suspended solids are varied, ranging from clay, silt, and plankton, to industrial wastes and sewage.
At higher levels of turbidity, water loses its ability to support a diversity of aquatic organisms. Waters become warmer as suspended particles absorb heat from sunlight proportionate to the concentration of particles.
Remember, warm water holds less oxygen than cooler water. Less light penetrating the water decreases photosynthesis, which in turn reduces oxygen concentrations. Suspended solids may clog fish gills, reduce growth rates and decrease resistance to disease, as well as preventing egg and larval development. Particles of silt, clay, and organic materials may settle to the river bottom, especially in impounded and slow-moving rivers. These settled particles can accumulate and smother eggs of fish and aquatic insects resting on the river bottom. Newly-hatched insect larvae may also be suffocated by the accumulated material. This material also settles into the spaces between rocks making these microhabitats unsuitable for mayfly nymphs, stonefly nymphs, caddisfly larvae, and other aquatic insects living there.
Turbidity may be the result of soil erosion, waste discharge, urban runoff, abundant bottom feeders (carp) which stir up bottom sediments, or the presence of excess nutrients which result in algal growth. Turbidity may affect the colour of the water, from nearly white to red-brown, as well as green from algal blooms.
Turbidity is a measure of water’s lack of clarity. Water with high turbidity is cloudy, while water with low turbidity is clear. The cloudiness is produced by light reflecting off of particles in the water; therefore, the more particles in the water, the higher the turbidity. Many factors can contribute to the turbidity of water.
An increase in stream flow due to heavy rains or a decrease in stream-bank vegetation can speed up the process of soil erosion. This will add suspended particles, such as clay and silt, to the water.
Runoff of various types contains suspended solids that may add to the turbidity of a stream. Agricultural runoff often contains suspended soil particles. Other types of runoff include industrial wastes, water treatment plant effluent, and urban runoff from parking lots, roads, and rooftops. Bottom-dwelling aquatic organisms, such as catfish, can contribute to the turbidity of the water by stirring up the sediment that has built up on the bottom of the stream. Organic matter such as plankton or decaying plant and animal matter that is suspended in the water can also increase the turbidity in a stream.
High turbidity will decrease the amount of sunlight able to penetrate the water, thereby decreasing the photosynthetic rate. Reduced clarity also makes the water less aesthetically pleasing. While this may not be harmful directly, it is certainly undesirable for many water uses. When the water is cloudy, sunlight will warm it more efficiently. This occurs because the suspended particles in the water absorb the sunlight, warming the surrounding water. This can lead to other problems associated with increased temperature levels. While highly turbid water can be detrimental to an aquatic ecosystem, it is not correct to assume that clear water is always healthy. Slightly turbid water can be perfectly healthy, while clear water could contain unseen toxins or unhealthy levels of nutrients.
Expected Levels
Turbidity is measured in NephelometricTurbidity Units, NTU. According to the USGS, the turbidity of surface water is usually between 1 NTU and 50 NTU. Turbidity is often higher than this, however, especially after heavy rain when water levels are high. Turbidity can be lower than expected in still water because of the settling of suspended particles that might occur. Water is visibly turbid at levels above 5 NTU. The standard for drinking water is 0.5 NTU to 1.0 NTU.
Summary of Method
Turbidity is measured using a Vernier Turbidity Sensor. A cuvette of sample water is placed in the Turbidity
Sensor and the value in NTU is read directly from the computer. Measurements can be made on site or in the lab at a later time.
Turbidity Testing Procedure
1.
Gently invert the sample water to mix in any particles that may have settled to the bottom. Important: Do not shake the sample. Shaking will introduce tiny air bubbles that will affect turbidity.
2.
Empty the water from the cuvette
3.
Rinse the cuvette with sample water, and then fill it with sample water so that the bottom of the meniscus is even with the top of the white line. Place the lid on the cuvette. Gently wipe the outside with a soft, lintfree cloth or tissue.
4.
Check the cuvette for air bubbles. If air bubbles are present, gently tap the bottom of the cuvette on a hard surface to dislodge them.
5.
Holding the cuvette by the lid, place it into the Turbidity Sensor. Make sure it is in the same orientation in the cuvette slot that it was before. Close the lid of the Turbidity Sensor.
6.
Keep the lid closed for the next 10 seconds. Note: Particles in the water will settle over time and show a slow downward drift in turbidity readings. Therefore, start data collection soon after placing the cuvette in the sensor.
7.
When the sampling run is complete, record the reading
8.
Return to Step 1 to obtain a second reading. When both readings have been taken, rinse the cuvette with distilled water.
TOTAL SOLIDS
Total Solids Background Information
This water quality measure (also referred to as total residue) includes: 1) dissolved solids (filterable residue) or that portion of the materials in water which pass through a filter and 2) suspended solids (non-filterable residue) which is that portion of the total retained by a filter.
Dissolved or inorganic materials include calcium, bicarbonate, nitrogen, phosphorous, iron, sulphur, and other ions found in a water body. A constant level of these materials is essential for the maintenance of aquatic life because the density of total solids determines the water balance. This affects the flow of water in and out of organisms’ cells. Also, many of these dissolved ions such as nitrogen, phosphorous, and sulphur are building blocks of molecules necessary for life. Suspended solids include silt and clay particles from soil runoff, plankton, industrial wastes, and sewage.
High concentrations of total solids cause lower water quality and water regulations problems for individual organisms. Low concentrations may limit the growth of aquatic life, or restrict some organisms from surviving in the water. Phytoplankton, for example, are totally dependent upon nitrates and phosphates that are dissolved in the water.
High concentrations of dissolved solids can lead to laxative effects in drinking water and impart an unpleasant mineral taste to the water. High concentrations of suspended solids also reduces with toxic compounds and heavy metals, and can lead to an increase in water temperature through greater absorption of sunlight by surface waters.
Outside sources that can affect the natural balance of total solids include urban runoff, such as salt from the streets in winter, or fertilizers from residential and agrianthropogenic use (composed mainly of phosphorous and nitrogen). Phosphorous and nitrogen may also be discharged from wastewater treatment plants. Sources that can affect the level of suspended solids are leaves and other plant materials entering the river (which are broken down into particles of organic matter); suspended sediments (clay particles) from urban runoff and soil erosion, and decayed plant and animal matter (converted to particulate matter within the river) and water leached from refuse sites.
Total solids, TS, is a measure of all the suspended, colloidal, and dissolved solids in a sample of water. This includes dissolved salts such as sodium chloride, NaCl, and solid particles such as silt and plankton. An excess of total solids in rivers and streams is a very common problem. The Environmental Protection Agency’s National
Water Quality Inventory1 has concluded that siltation, one of the primary contributors to total solids, is the most common pollutant of streams and rivers they sampled.
Many factors can contribute to the total solids in water. Soil erosion is a large contributor. An increase in water flow or a decrease in stream-bank vegetation can speed up the process of soil erosion and contribute to the levels of suspended particles such as clay and silt. Naturally occurring rocks or minerals in the soil such as halite, NaCl, or limestone, CaCO3, may also dissolve into the water, adding to the total solids.
Total solids can also come from various types of runoff. Agricultural runoff often contains fertilizers and suspended soil particles. Other sources include industrial wastes, effluent from water treatment plants, and urban runoff from parking lots, roads, and rooftops. Bottom-dwelling aquatic organisms, such as catfish, can contribute to the total solids in the water by stirring up the sediment that has built up on the bottom of the stream. Organic matter such as plankton or decaying plant and animal matter that are suspended in the water will also add to the total solids in a stream.
Dissolved solids often make a significant contribution to the amount of total solids in water. In fact, the mass of the dissolved solids is sometimes higher than the mass of the suspended particles. Dissolved solids in
freshwater samples include soluble salts that yield ions such as calcium, chloride, bicarbonate, nitrates, phosphates, and iron.
If the levels of total solids are too high or too low, it can impact the health of the stream and the organisms that live there. High levels of total solids will reduce the clarity of the water. This decreases the amount of sunlight able to penetrate the water, thereby decreasing the photosynthetic rate. Reduced clarity also makes the water less aesthetically pleasing. While this may not be harmful directly, it is certainly undesirable for many water uses. When the water is cloudy, sunlight will warm it more efficiently. This occurs because the suspended particles in the water absorb the sunlight which, in turn, warm the surrounding water. This leads to other problems associated with increased temperature levels.
As previously mentioned, dissolved solids often make a large contribution to total solids. The correct balance of dissolved solids in the water is essential to the health of aquatic organisms for several reasons. One reason is that many of these dissolved materials are essential nutrients for the general health of aquatic organisms. Another reason is that the transport of ions through cellular membranes is dependent on the total
Ionic strength of the water. Too many dissolved salts in the water can dehydrate aquatic organisms. Too few dissolved salts, however, can limit the growth of aquatic organisms that depend on them as nutrients.
Expected Levels
Total solids in surface water usually fall within the range of 20 mg/L to 500 mg/L. Values can go much higher especially after heavy rain when the water levels are high.
Summary of Method
You will determine the total solids in a sample of water by adding a precise amount of water to a carefully cleaned, dried, and weighed beaker. The water is then evaporated away using a drying oven and the beaker is reweighed. The difference in mass before and after is the mass of the total solids. Calculations are made to convert the change in mass to mg/L total solids.
Procedure - TOTAL SOLIDS
Equipment
Sample container
250ml beaker per sample
Desiccator with dry desiccating beads (the beads should be blue – not pink)
Electronic balance capable of measuring to 3 decimal places
100ml measuring cylinder
Hotplate
Tongs
NB The hotplates must not be left unattended during use
1.
Pre-dry the required beakers on a hotplate on a medium setting for 5-10 minutes.
This may take some time so some members of the group may perform other tests or write up work during this time.
2.
Using tongs, place the beaker into the desiccator and leave for 10-15minutes to cool.
This may take some time so some members of the group may perform other tests or write up work during this time.
3.
Remove the beakers required from the desiccator with tongs and accurately weigh and record the weight of the beaker.
4.
Accurately measure 100ml of sample and pour into the pre-weighed beaker.
5.
Place the beaker on the hotplate and boil dry. Once the beaker is almost dry, turn the hotplate setting onto low and heat until completely dry. Once it appears to be dry, leave it heating for a further two minutes.
6.
Remove beaker from hotplate and transfer to the desiccator to cool using tongs.
7.
When cool, remove from the desiccator and reweigh.
Increase in weight (g) = Final weight – Initial weight
Total Solids mg/L = Increase in weight x 1 000 000
Volume of sample (ml)
8.
If the mass of the solids is less than 0.025 g, add another 100 mL of sample to each beaker and repeat
Steps 5-7. Make a note that your total volume is now 200 mL instead of 100 mL.
9.
Record the mass of the solids (round to the nearest 0.001g)
10.
Determine the Q value using the result above.
DISSOLVED OXYGEN (D.O.)
Background Information
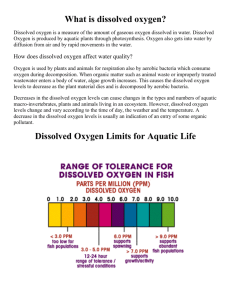
Dissolved oxygen is an essential element for the maintenance of healthy lakes and rivers. Most aquatic plants and animals need oxygen dissolved in the water for survival. Some aquatic organisms like barramundi and mayfly larvae require medium-to-high levels of dissolved oxygen to live. Other aquatic organisms like carp and catfish flourish in waters of low dissolved oxygen. The dissolved oxygen level of water is one indicator of the health of aquatic ecosystems. Water of consistently high dissolved oxygen levels are usually considered healthy and stable aquatic ecosystems capable of supporting many different kinds of aquatic organisms.
Sudden and gradual depletions in dissolved oxygen can cause major shifts in the kinds and diversity of aquatic organisms-from pollution intolerant species to pollution tolerant species and from high organism diversity to low organism diversity. With a drop in dissolved oxygen levels many different kinds of aquatic insects—mayfly nymphs, stonefly nymphs, caddis fly larvae, and beetle larvae—might be reduced to a few different kinds, such as aquatic worms and fly larvae that are tolerant of these levels. Nuisance algae and anaerobic organisms (which live without oxygen) may also become abundant in waters of low dissolved oxygen.
Oxygen saturated waters have a pleasant taste and waters lacking in dissolved oxygen have and insipid taste.
But what causes depletions in dissolved oxygen?
The main factor is the accumulation of organic wastes or wastes primarily derived from once-living plants and animals and from the faeces of animals. Organic waste takes the form of sewage; urban and agricultural runoff; discharge of food processing industries, meatpacking plants, and dairies; and natural sources like leaves falling into the river and decaying aquatic plants and animals. Aerobic bacteria consume oxygen in the process of decomposing or breaking-down these organic wastes.
What are the sources of dissolved oxygen in water?
Much of the dissolved oxygen in water comes from the atmosphere. Atmospheric oxygen enters water more readily when mixing occurs, as in waves on lakes, dams or slow-moving rivers, and in fast-moving rivers that tumble over rocks and gravel. Another major source of dissolved oxygen is photosynthesis by algae and larger aquatic plants.
In lakes, dams and large slow-moving river reaches, algae and larger aquatic plants are more abundant than in rivers with significant current. From morning through the afternoon hours, dissolved oxygen levels rise through photosynthesis. Late in the afternoon, dissolved oxygen levels are highest. As the sun sets, photosynthesis stops, but plant and animal respiration continues to consume oxygen. Just before dawn, dissolved oxygen levels fall to their lowest level. Large fluctuations in dissolved oxygen from late afternoon to early morning are characteristic of lakes and impounded and slow-moving rivers with extensive plant growth. Dissolved oxygen levels may fall below 4 mg/l in such water.
What are some natural factors that may affect dissolved oxygen levels particularly in rivers?
These factors include; temperature, flow, season, and the physical structure of the river. Cold water can hold more oxygen than warmer water because gases are more soluble in cooler water. Flow velocity and season can be related in some rivers. During dry periods (summer), river flow is reduced. In some rivers this reduction in flow velocity means less mixing of the water with the atmosphere and consequently lower dissolved oxygen levels.
Organic waste entering rivers with reduced flow exert a great demand on available dissolved oxygen. If the physical structure of the river is altered through damming, current velocity is drastically reduced and less mixing of the water with the atmosphere occurs. Dammed river reaches may support extensive aquatic plant growth and collect organic materials washing in from upriver.
How is the level of dissolved oxygen present in water at a given temperature determined?
It is determined through the use of the percent saturation value. Rivers with a constant 90 percent dissolved oxygen saturation value or above are considered healthy. Rivers below 90 percent saturation may have larger amounts of oxygen-demanding materials (organic wastes).
To use the chart, draw a straight line between the water temperature at the test site and the dissolved oxygen measurement (oxygen mg. per litre), and read the saturation percentage at the intercept on the sloping scale.
Dissolved Oxygen Sampling Procedure
It is best to sample away from shore and midway between the surface and bottom. In free-flowing rivers with good mixing any samples taken beneath the surface and in the current will probably be representative samples.
In slow-moving river reaches and in dam areas it is very important to sample away from shore and midway between the surface and bottom, or to sample at various depths. In slow-moving rivers and dams with little mixing, shore sampling will probably not provide a representative sample. Aquatic vegetation in these slow current areas produces oxygen near the surface. Decaying vegetation on the bottom consumes oxygen through the respiration of aerobic (requires oxygen) bacteria that decompose this vegetation.
Ensure the bottle is completely full and place stopper in the mouth. Water should overflow out of the bottle and no air bubbles should be trapped in the bottle. If large air pockets are trapped in the bottle, empty the bottle and resample. A temperature measurement should also be taken at this time so that %oxygen levels can be calculated.
Remember that the dissolved oxygen test should be run immediately after sampling.
Procedure
Dissolved Oxygen in Water – using Vernier Data logger
Although water is composed of oxygen and hydrogen atoms, biological life in water depends upon another form of oxygen—molecular oxygen. Oxygen is used by organisms in aerobic respiration, where energy is released by the combustion of sugar in the mitochondria. This form of oxygen can fit into the spaces between water molecules and is available to aquatic organisms. Fish, invertebrates, and other aquatic animals depend upon the oxygen dissolved in water. Without this oxygen, they would suffocate. Some organisms, such as salmon, mayflies, and trout, require high concentrations of oxygen in their water. Other organisms, such as catfish, midge fly larvae, and carp can survive with much less oxygen. The ecological quality of the water depends largely upon the amount of oxygen the water can hold. The following table indicates the normal tolerance of selected animals to temperature and oxygen levels. The quality of the water can be assessed with fair accuracy by observing the aquatic animal populations in a stream.
Table 1
Animal
Trout
Smallmouth bass
Caddisfly larvae
Mayfly larvae
Stonefly larvae
Catfish
Carp
Mosquito
Water boatmen
Temperature Range (°C)
5–20
5–28
10–25
10–25
10–25
20–25
10–25
10–25
10–25
Minimum Dissolved Oxygen (mg/L)
6.5
6.5
4.0
4.0
4.0
2.5
2.0
1.0
2.0
MATERIALS
Computer 100 mL beaker Vernier computer interface
Vernier Dissolved Oxygen Probe hot and cold water two 250 mL beakers
Vernier Temperature Probe 1 L plastic milk container Logger Pro Styrofoam cup
Expected Levels
The unit mg/L2 is the quantity of oxygen gas dissolved in one litre of water. When relating DO measurements to minimum levels required by aquatic organisms, mg/L is used. The procedure described in this chapter covers the use of a Dissolved Oxygen Probe to measure the /concentration of DO in mg/L. Dissolved oxygen concentrations can range from 0 to 15 mg/L. Cold mountain streams will likely have DO readings from 7 to 15 mg/L, depending on the water temperature and air pressure. In their lower reaches, rivers and streams can have DO readings between 2 and 11 mg/L.
When discussing water quality of a stream or river, it can be helpful to use a different unit than mg/L. The term percent saturation is often used for water quality comparisons. Percent saturation is the dissolved oxygen reading in mg/L divided by the 100% dissolved oxygen value for water (at the same temperature and air pressure). The manner in which percent saturation relates to water quality is displayed in Table 2. In some cases, water can exceed 100% saturation and become supersaturated for short periods of time.
Summary of Methods
Dissolved oxygen can be measured directly at the site or from water samples transported from the site.
Measurements can be made at the site by either placing the Dissolved Oxygen Probe directly into the stream away from the shore or by collecting a water sample with a container or cup and then taking measurements with the Dissolved Oxygen Probe back on the shore. Water samples collected from the site in capped bottles and transported back to the lab must be stored in an ice chest or refrigerator until measurements are to be made.
Transporting samples is not recommended, because it reduces the accuracy of test results.
Method
1. Rinse the tip of the probe with sample water.
2. Place the tip of the probe into the stream at Site 1, or into a cup with sample water from the stream.
Submerge the probe tip to a depth of 4-6 cm.
3. Click to begin data collection.
4. Gently stir the probe in the water sample. Important: Leave the probe tip submerged for the 10 seconds that data is being collected.
5. When the sampling run is complete, stop data collection and record the mean dissolved oxygen concentration value sheet.
6. Return to Step 1 to obtain a second reading. When both readings have been taken, rinse the tip of the probe and secure it in the calibration bottle filled with water.
BIOCHEMICAL OXYGEN DEMAND (B.O.D)
Background Information
Biochemical oxygen demand (BOD) measures the amount of organic material in water. Organic matter is fed upon by aerobic bacteria which require oxygen. In this process organic matter is broken down and oxidized
(combined with oxygen). Protozoa (like Paramecium) prey upon the growing population of bacteria and also require oxygen. Biochemical oxygen demand uses this process as a measure of organic material in the water by analysing the quantity of oxygen used by these microorganisms in the aerobic oxidation of organic matter.
B.O.D is a measure of the
Organic Matter
Bacteria break down the
Organic Matter
Dissolved oxygen consumed by bacteria over 5 in the water
So:
More O.M. = more bacteria = more oxygen consumed over 5 days
days
The time taken for the oxidation of organic matter is dependent on the amount of micro-organisms present and the initial amount of food (organic matter) available will determine the rate of biological growth. This can be demonstrated by the biological growth curve. In the initial stages of growth excess food and nutrients are present so that reproduction is unrestricted and numbers increase at an exponential rate. At some point the concentration of food or a particular nutrient becomes limiting and growth of the microorganisms is increasingly restricted. In the final stage all the food has been consumed so that growth ceases and the numbers of micro-organisms decrease. Organic matter from the dead cells is utilised by the remaining organisms in auto-oxidation or endogenous respiration. This curve is basically true for any biological population, including that present in the BOD sample. Laboratory tests of many types of samples has led to the standard of
5 days incubation being adopted as this is the time most samples pass the “declining growth” stage.
Where does this organic matter come from?
There are natural sources of organic material which include organic matter entering lakes and rivers from swamps, bogs, and vegetation along the water (particularly leaf fall). There are also human-based sources of organic matter contributing organic matter above natural levels. These sources of organic matter will be referred to as organic pollution.
Point sources of organic pollution come from identifiable points such as:
Pulp and paper mills;
Meat-packing plants;
Food processing industries; and,
Wastewater treatment plants.
Non-point pollution arises from multiple sources that are difficult to identify. Non-point sources of organic pollution include urban and agricultural runoff from the following sources:
Pet wastes from streets and sidewalks; nutrients from lawn fertilizers; leaves; grass clippings, and paper from residential areas;
Nutrients, like nitrogen and phosphates carried from eroded fields by runoff.
Runoff from animal feedlots.
How does an influx of nutrients, like nitrates and phosphates, into a river contribute to a high biochemical oxygen demand?
As explained in the dissolved oxygen section, decaying aquatic plants and their associated decomposers (aerobic bacteria), demand oxygen during plant decomposition. Nutrient input into the river—from nitrates and phosphates—will stimulate plant growth. Eventually, more plant growth leads to more plant decay. Therefore nutrients can be a major force in high biochemical oxygen demand in rivers. Abundant plant growth is much more likely to occur in dams and in slow reaches of free-flowing rivers. Dams also collect organic wastes from upriver, which settle in this quieter water; the bacteria that feed on this organic waste consume oxygen.
Percent saturation (dissolved oxygen) values in waters with much plant growth and decay will probably fall below
90 percent.
What effect might a high biochemical oxygen demand have upon the types and diversity of aquatic organisms?
Organisms that are more tolerant of lower dissolved oxygen will appear and proliferate—such as carp, midge larvae, and sewage worms. Other organisms that are intolerant of low dissolved oxygen levels will not survive in this water – such as caddisfly larvae, mayfly nymphs, and barramundi. In these waters of high biochemical oxygen demand, a low diversity of aquatic organisms will replace the ecologically stable and complex associations present in waters containing a high diversity of organisms.
Expected Levels
BOD levels are dependent on the body of water being tested. Shallow, slow-moving waters, such as ponds and wetlands, will often have large amounts of organic material in the water and high BOD levels. A water sample from a pond could have an initial dissolved oxygen reading of 9.5 mg/L. After the five-day incubation period, the dissolved oxygen could be down to 1 mg/L resulting in a high BOD level of 8.5 mg/L. In contrast, a water sample collected from a cold mountain stream with an initial dissolved oxygen reading of 11 mg/L may have decreased to 9 mg/L after incubation, resulting in a BOD of only 2 mg/L. Use Table 1 as a rough guide for the data you gather.
Table 1: Interpretation of BOD Levels
Summary of Methods
Included in this test are the procedures for High and Low BOD levels. Decide beforehand, based on expected
BOD levels (see Table 1), which procedure is appropriate for the water you are testing. Only one of the two tests should be performed.
Method 1: Low BOD Levels (0–6 mg/L)
BOD is calculated from two separate dissolved oxygen measurements made using the Dissolved Oxygen Probe.
The initial dissolved oxygen reading is taken at the sampling site using the procedures outlined in DO. Using a light-free sample bottle, a water sample is collected at the same site, wrapped in alfoil. The sample is transported back to the lab and incubated at 20°C for a total of five days. After five days, the incubated sample is tested for dissolved oxygen. The oxygen reading at the end of the five days is subtracted from the initial reading. The resulting value is the BOD level.
Method 2: High BOD Levels (> 6 mg/L)
This method is recommended when testing stagnant or polluted waters, in which all of the dissolved oxygen may be consumed before the end of the 5-day period. The initial dissolved oxygen test, sampling, storage and incubation, are performed in the same manner as found in Method 1. Differences for Method 2 are:
• Five water samples are collected.
• A sample is tested for dissolved oxygen every 24 hours for five days.
• If, before the fifth day, the dissolved oxygen concentration falls below 4.0 mg/L, oxygen is added to the remaining samples by aeration.
• Add each bottle’s change in dissolved oxygen concentration to obtain the BOD value.
TOTAL PHOSPHORUS
Total Phosphorus Background information
Total Phosphorus includes organic phosphorous and inorganic phosphate. Organic phosphorous is a part of living plants and animals. It is attached to particulate organic matter composed of once-living plants and animals.
Inorganic phosphates comprise the ions (H
2
PO
4
, HPO
4
and PO
4
) bonded to soil particles, and phosphates present in laundry detergents (polyphosphates).
Phosphorus is an essential element for life; it is a plant nutrient needed for growth and a fundamental element in metabolic reactions of plants and animals. Plant growth is limited by the amount of phosphorus available. In most waters, phosphorus functions as a “growth-limiting” factor because it is usually present in very low concentrations. This scarcity of phosphorus can be attributed to its relationship with organic matter and soil particles. Any unattached of “free” phosphorus, in the form of inorganic phosphates, is rapidly taken up by algae and larger aquatic plants. Because algae only require small amounts of phosphorus to live, excess phosphorus causes extensive algal growth called algal blooms. Algal blooms colour the water pea-soup green and is a classic symptom of anthropogenic eutrophication.
Anthropogenic eutrophication is an enrichment of the water, usually by phosphorus, from human activities.
Natural eutrophication is slight because phosphorus becomes trapped in bottom sediments in deeper water, and any free phosphorus is rapidly taken up by aquatic plants. Forest fires and fallout from volcanic eruptions are natural occurrences which release phosphorus and may cause eutrophication. Lakes that receive no inputs of phosphorus from human activities age extremely slowly. Most of the eutrophication occurring today is anthropogenic eutrophication.
The physical nature of the lake or river impoundment influences how sensitive these waters are to humanderived inputs of phosphorus. Naturally shallow lakes and impoundments show the effects of anthropogenic eutrophication more readily; aquatic vegetation may completely cover the surface of these waters. Rooted aquatic plants can draw phosphorus previously locked within bottom sediments and release this phosphorus into the water, intensifying eutrophication. Eventually, the entire lake or impoundment will fill with aquatic vegetation. Eutrophication is not as great a problem in free-flowing rivers because rooted aquatic plants are more abundant in still water and nutrients do not accumulate as they do in still water.
What are the common sources of phosphorus which cause anthropogenic eutrophication in lakes and river impoundments?
Phosphorus is derived from several sources: human wastes, animal wastes, industrial waste, and through human disturbance of the land and its vegetation.
Inadequately treated sewage from wastewater treatment plants and septic tanks are responsible for some phosphorus input to the water. Sewage effluent should not contain more than 1 mg/l phosphorus according to the Environmental Protection Agency, but outdated wastewater treatment plants often fail to meet this standard. Some industrial wastes may not be adequately treated to remove chemicals which interfere with phosphorus removal at the wastewater treatment plant. Animal wastes, which contain phosphorus, find their way into lakes and rivers via rainfall that runs off feedlots and barnyards.
Removal of natural vegetation, for farming and construction of roads and buildings, strips the soil cover which previously held phosphorus, Rain and melting snow further erodes this exposed soil, contributing to phosphorusladen runoff into lakes and rivers.
Farming and home building also creates a market for crop and lawn fertilizers which contain phosphorus; this phosphorus finds its way into watershed lakes and rivers from surface runoff.
Draining swamps and marches from farmland of shopping malls releases nutrients like phosphorus which have remained dormant in years of accumulated organic deposits. Also, these drained wetlands no longer function as filters of silt and phosphorus from floodwaters and other runoff from the land.
What are the effects of anthropogenic eutrophication upon lake or river impoundment?
The first symptom of anthropogenic eutrophication is an algal bloom which colours the water a pea-soup green.
As eutrophication increases, algal blooms become more frequent. In shallow areas; rooted aquatic plants which normally grow there become very dense; swimming and boating becomes impractical ion this area.
You are aware that this luxuriant plant growth produces supersaturated (extremely high) dissolved oxygen conditions during the day; however, at night the dissolved oxygen levels will drop due to plant decomposition and plant and animal respiration. This growth of algae and larger aquatic plants will generate more plant decomposition, and decomposer organisms like aerobic bacteria will demand more oxygen. As greater amounts of plants die and decompose, more dissolved oxygen is consumed; water in advanced stages of anthropogenic eutrophication can become anaerobic (without oxygen). Anaerobic conditions usually occur near the lake or impoundment bottom and produce gases like hydrogen sulphide which foul the shoreline with a “rotten egg” smell.
What happens to aquatic organisms under these eutrophic conditions?
A lake or impoundment which previously supported only support carp and other tolerant organism’s under eutrophic conditions. Organism diversity decreases in very eutrophic lakes. The many different species that exist in clean water are replaced by few different kinds of organisms which can tolerate low dissolved oxygen levels—carp, midge larvae, sewage worms (Tubifex), and others.
Those people who have homes or cottages on eutrophic lakes and river impoundments will see their property values decline. These eutrophic waters become unfit for recreation, and become unattractive.
Are there ways to reverse the effects of anthropogenic eutrophication?
Yes. A good example of a lake which was once highly eutrophic but whose water quality is improving is Lake Erie.
Aquatic ecosystems have the capacity to recover is the opportunity is provided by:
1.
reducing or eliminating our use of lawn fertilizers that daring into waterways;
2.
encourage farmers to practice low-till farming in order to reduce soil erosion. All farmers should test their soil so the amount of fertilizer applied can be matched with soil needs; this action would decrease phosphorus runoff. Farms with cattle (dairy and beef farms) should build storage areas or collecting areas for feedlots so that surface runoff;
3.
preserving natural vegetation whenever possible, particularly near shorelines. Preservation of wetlands is vital in order to absorb nutrients and maintain water levels.
4.
supporting measures (including taxes) to improve phosphorus removal by wastewater treatment plants and septic systems. Also, treatment of storm sewer wastes may be necessary to limit phosphorus inputs to rivers and lakes. Homeowners may need to consider investing in a community sewer system; and
5.
requiring particular industries to prevent their wets sending it to wastewater treatment plant.
Procedure HI 3833 – Phosphate Test Kit
1.
Remove the cap from the plastic vessel. Rinse the plastic vessel with the water sample, fill it to 10 mL mark
2.
Add 1 packet of reagent HI 3833-0
3.
Replace the cap and mix solution until solids dissolve
4.
Remove the cap and transfer the solution into the colour comparator cube. Let set for 1 minute.
5.
Determine which colour matches the solution in the cube and record the result as mg/L (ppm) of phosphate (PO
4
3-)
NITRATES
Nitrates Background Information
Nitrogen is a fundamental plant nutrient and required by all living plants and animals for building protein. In aquatic ecosystems, nitrogen is present in many different forms. Nitrogen is most abundant in its molecular form (N
2
) which makes up 79 percent of the air we breathe. It is a much more abundant nutrient than phosphorous in nature. In its molecular form (N
2
), nitrogen is useless for most aquatic plant growth.
Blue-green algae, the primary algae of algal blooms, are able to use the molecular form of nitrogen (N biologically convert it to usable forms of nitrogen for aquatic plant growth, ammonia (NH
3
2
) and
) and nitrates (NO
3
).
If plants are able to take up ammonia and nitrates through their roots, how do aquatic organisms obtain the nitrogen they need to form proteins? Aquatic organisms eat aquatic plants and covert plant proteins to specific animal proteins or they eat other aquatic organisms which feed upon plants.
As aquatic plants and animals die, bacteria breakdown large protein molecules into a final product, ammonia.
Ammonia is then oxidized (combined with oxygen) by specialized bacteria to form nitrates (NO
2
) and nitrates
(NO
3
). These bacteria derive energy for metabolism from oxidization. Excretions of aquatic organisms are very rich in ammonia, although the amount of nitrogen they ass to waters is usually small. Duck and geese, however, contribute a heavy load of nitrogen (from excrement) in areas where they are plentiful. Through decomposition of dead plants and animals and excretions of living animals, nitrogen that was previously “lockedup” is now released.
There are even bacteria that can transform nitrates (NO
3
) into free molecular nitrogen (N
2
) which can be lost to the atmosphere. The nitrogen cycle begins again if this molecular nitrogen is converted by blue-green algae into ammonia and nitrates.
Do high nitrogen levels also contribute to eutrophication?
Because nitrogen, as ammonia and nitrates, acts as a plant nutrient, it also causes eutrophication. Nitrogen rarely limits plant growth (unlike phosphorous), so plants are not as sensitive to increases in ammonia and nitrate levels.
As you read in the Total Phosphorous section, eutrophication causes more plant growth and decay which exerts a biochemical oxygen demand.
What are the human sources of nitrate input into lakes and rivers?
These are inadequately treated sewage from wastewater treatment plants; runoff that flushes storm drains containing illegal sanitary sewer connections; and, poorly functioning septic systems.
Septic systems are a very common treatment method around rural areas. Instead of a large, centralised sanitary sewer to which everyone hooks into in the city, people with septic systems have individual sewer systems. A septic system is comprised of a main pipe from the house to a box called a septic tank. These pipes are arranged in a grid which usually lies over stone and gravel and is called a “drainfield”. Wastes from the toilet, kitchen sink, bathtub, and washing machine flow through an underground pipe to a septic tank. In the septic tank, large wastes settle and floating grease is skimmed off. The remaining liquid effluent enters the drainfield and is forced out holes in the pipe to trickle through stone, gravel and soil. In properly functioning septic systems, soil particles remove nutrients like nitrates and phosphates before they reach the groundwater. Along some lakes and rivers, septic system drainfields are too near the water table. This reduces the depth of soil and its capacity to remove nitrates and phosphorous. These nutrients find their way into the lake or river impoundment via ground water flow. People who neglect septic tank maintenance may allow
their tanks to fill with solid materials from previous settling. When the tank is full, household wastes go directly to the drainfield instead of settling in the tank. These unsettled wastes can plug the drainfield, which prevents filtering of the liquid wastes. In this condition, household sewage may start to pool on the ground and enter via storm runoff.
Poorly functioning septic systems located close to a well may contaminate groundwater with nitrates. Water containing high nitrate levels, if used for infant milk formula, can cause a condition called methemoglobinemia
(met-hemo-glo bin-emia). This condition prevents the baby’s blood from carrying oxygen, hence the nickname
“blue-babies”. High nitrate levels have also been discovered in groundwater underneath croplands; excessive fertilizer use seems to be the cause, especially in healthy irrigated areas with sandy soils.
Ground level
Septic Tank
Figure 25. Septic system.
Stormwater runoff containing nitrates from lawn and crop fertilizers are responsible for some enrichment of receiving waters. Also, runoff from feedlots and barnyards containing concentrated amounts of ammonia and nitrates.
As discussed in the Total Phosphorous section, people have created the eutrophication problem which threatens to limit organism diversity, recreational opportunities, and property values—only we can reverse eutrophication through thoughtful action.
The tests described here are used to measure the concentration of nitrate ions, NO3 –, in a water sample. The concentration of nitrate will be expressed throughout this section in units of mg/L NO3 –-N. The unit, NO3
–-N, means simply “nitrogen that is in the form of nitrate.” Nitrate ions found in freshwater samples result from a variety of natural and manmade sources. Nitrates are an important source of nitrogen necessary for plants and animals to synthesize amino acids and proteins. Most nitrogen on earth is found in the atmosphere in the form of nitrogen gas, N2. Through a process called the nitrogen cycle, nitrogen gas is changed into forms that are useable by plants and animals. These conversions include industrial production of fertilizers, as well as natural processes, such as legume-plant nitrogen fixation, plant and animal decomposition, and animal waste.
Although nitrate levels in freshwater are usually less than 1 mg/L, manmade sources of nitrate may elevate levels above 3 mg/L. These sources include animal feedlots, runoff from fertilized fields, or treated municipal wastewater being returned to streams. Levels above 10 mg/L in drinking water can cause a potentially fatal disease in infants called methemoglobinemia, or Blue-Baby Syndrome. In this disease, nitrate converts haemoglobin into a form that can no longer transport oxygen.
High nitrate concentrations also contribute to a condition in lakes and ponds called eutrophication, the excessive growth of aquatic plants and algae. Unpleasant odour and taste of water, as well as reduced clarity, often accompany this process. Eventually, dead biomass accumulates in the bottom of the lake, where it decays and compounds the problem by recycling nutrients. If other necessary nutrients are present, algal blooms can occur in a lake with as little as 0.50 mg/L NO3 –-N.
Nitrate pollution of surface and groundwater has become a major ecological problem in some agricultural areas.
Although fertilizer in runoff is most often blamed, there is evidence that concentration of livestock in feedlots is now the major source of agricultural nitrate pollution. Runoff from fertilized fields is still a significant source of nitrate, although fertilizer use peaked in 1981 and has remained fairly constant since.
Expected Levels
The nitrate level in freshwater is usually found in the range of 0.1 to 4 mg/L NO3 –-N. Unpolluted waters generally have nitrate levels below 1 mg/L. The effluent of some sewage treatment plants may have levels in excess of 20 mg/L.
Summary of Methods
Method 1: Nitrate Ion-Selective Electrode
A Vernier Nitrate Ion-Selective Electrode (ISE) is used to measure the nitrate-ion concentration in the water, in mg/L NO3 –-N, either on site or after returning to the lab.
Procedure – Nitrates
1.
Rinse the ISE with distilled water and gently blot it dry.
2.
Place the tip of the ISE into the stream at Site 1, or into a cup with sample water from the stream.
Make sure the ISE is not resting on the bottom and that the small white reference contacts are immersed. Make sure that no air bubbles are trapped below the ISE.
3.
Important: Leave the probe tip submerged for the 10 seconds that data is being collected.
4.
When the sampling run is complete, record the mean nitrate concentration value.
5.
Return to Step 1 to obtain a second reading
HI 3874 Nitrate Test Kit
Significance and Use
Nitrate ions are present in trace amounts in surface water and in higher levels in some groundwater.
Nitrate is found only in small quantities in domestic wastewater but can reach higher concentration (up to 30 mg/L as nitrogen) in the outflow of nitrifying biological treatment plants. Excessive amounts can contribute to methaemoglobinemia: infant death and adult illness. In order to prevent this, a 10 mg/L limit (as nitrogen) has been imposed on drinking water.
NOTE: mg/L is equivalent to ppm (parts per million).
Chemical Reaction
Nitrates are reduced to nitrites in the presence of Cadmium. The nitrites thus produced react with the reagent to yield an orange compound. The amount of colour developed is proportional to the concentration of nitrate present in the aqueous sample.
Instructions
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
• Fill the glass cuvette with 10 mL of the sample, up to the mark
Add 1 packet of HI 3874-0 Nitrate Reagent.
• Replace the cap and shake vigorously for exactly 1 minute. A deposit may remain, but it will not affect measurement. Time and manner of shaking can affect the results.
• Wait for 4 minutes to allow the colour to develop. Remove the cap and fill the colour comparator cube with 5 mL of the treated sample (to the mark).
• Determine which colour matches the solution in the cube and record the result in mg/L (or ppm) of
Nitrate-nitrogen.
• It is better to match the colour with a white sheet at about 10 cm behind the comparator.
• To convert the reading to mg/L of Nitrate (NO3 -), multiply the reading by a factor of 4.43.
Safety Data Sheets
The chemicals contained in this kit may be hazardous if improperly handled. Read the relevant Safety
Data Sheet before performing this test.
FAECAL COLIFORM
Faecal Coliform Background Information
Bacteria are single-celled organisms that can only be seen with the aid of a very powerful microscope. Bacteria can be found everywhere- in air, water, soil, even in and on your own body. They can benefit us by recycling wastes, fixing nitrogen helping plants to grow, and by making certain types of food. They may harm us by causing diseases and food spoilage.
Bacteria reproduce rapidly if conditions are right for growth. Most bacteria grow best in dark, warm, moist environments with food. Some bacteria form colonies as they multiply which may grow large enough to be seen.
Tests for water Purity
Historically, most of our concern about water purity has been related to the transmission of disease.
Pathogenic organisms include bacteria, viruses, and parasites that cause diseases and illnesses. It is not practical however to look only for pathogens in the water supply as the Pathogens are relatively scarce in water, which makes them tedious and time-consuming to monitor directly an if and when they are finally detected, it may be too late to prevent a disease outbreak. Tests for water safety in use today are aimed at detecting particular indicator organisms. The criteria for an indicator organism include:
The indicator organism must be consistently present in human intestinal wastes in substantial numbers.
The indicator organism must be able to survive in the water at least as well as the more likely pathogenic organisms would.
The indicator organism must be detectable by simple tests.
In Australia, the usual indicator organisms are the faecal coliform bacteria. If faecal coliform bacteria are found to be present in large numbers (over 200 cfu/100ml water sample), it is assumed that contamination of the water body has occurred and that pathogens may be present. A person swimming in such water might swallow some pathogenic organisms, or these organisms might enter the body through the nose and ears or through a cut in the skin. Diseases and illnesses such as typhoid fever, hepatitis, gastroenteritis, dysentery, and ear infections can be contracted in waters with high faecal coliform counts. Additionally, if the faecal coliform levels are found to be low or zero, then the chance of pathogenic species such as Shigella spp, Vibrio
cholera or Salmonella spp being present is extremely low.
The simplest, most widely used test for coliform bacteria consists of filtering a water sample through a bacterial retentive membrane filter. The microscopically small filter pores let the water through, leaving microscopic life trapped on the filter surface. To count coliform bacteria, the analyst puts the filter on a suitable nutrient medium, and the nutrients wick up through the filter pores to keep the microbes fed and multiplying. The liquid nutrient also contains the lactose and a stain that advertises the presence of coliforms.
Within 24 to 36 hours, each microbe becomes a colony of billions of bacteria, visible even without a microscope.
Bacteria are too small to be counted as individuals, even with the use of a microscope, so the colonies are grown and counted and are expressed as colony forming units (cfu).
Drinking Water
Standards (cfu/1ml water)
1cfu/100 ml (total coliform*)
1 faecal coliform = 10 total coliform bacteria
Total Body Contact (Swimming)
Partial Body Contact (Boating)
Treated Sewage Effluent
200cfu/100 ml
1000cfu/100 ml
Faecal coliform levels shall not exceed 200 cfu/100 ml in treated sewage effluent.
*Total coliform includes bacteria from cold-blooded animals and various soil organisms.
The concentration of faecal coliform bacteria in water is measured to determine the likelihood of contamination by microbiological organisms. While faecal coliform are not pathogenic disease causing), they are commonly found alongside pathogenic organisms such as those responsible for dysentery, gastroenteritis, and hepatitis A. It is easier to test for faecal coliform than for pathogenic organisms; therefore, the presence of faecal coliform in a water sample is used to indicate potential contamination. A common source of coliforms and pathogenic bacteria is raw sewage. Faecal coliform bacteria occur naturally in the digestive tract of warm-blooded animals, where they aid in the digestion of food.
The results of coliform bacteria tests are generally used to monitor recreational areas, stormwater out-falls, and drinking water supplies. Water is commonly tested for three types of coliform bacteria: faecal coliforms, total coliforms, and E. coli. The standards for drinking water are generally based on total coliforms. The accepted standard for drinking water is that there should be no coliforms present after the water is filtered or treated. Natural waters will nearly always contain some form of bacteria. That is why you should never drink untreated water from a river or lake. Currently, the most common measurement for surface waters is faecal coliform. In some areas, standards for surface water contamination are shifting to the measurement of E. coli.
Expected Levels
Standards for faecal coliform differ from state to state. For specific requirements, it is best to contact your state or regional health department. Standards for faecal coliform are considerably stricter if the water is used for total body contact such as swimming, rather than used only for boating with minimal direct contact.
When interpreting data from faecal coliform tests, it is important to remember that there can be a high degree of randomness of distribution within a sample. A large number of data points are necessary to obtain statistically significant data. Faecal coliform is measured in colony forming units per 100 mL, CFU/100 mL, of water tested.
Table 11
Method - FAECAL COLIFORM
Sampling Procedure
If the purpose of sampling is to determine faecal coliform levels at a river reach, then samples should be taken beneath the water surface and in the current (if there is one). If the purpose of sampling is to confirm suspected sources of faecal coliform contamination, then samples should be taken just down river from the source (like the mouth of storm drain) and other samples taken upriver from the source for comparison.
There is also wet weather sampling and dry weather sampling. Wet weather sampling involves sampling, often at time intervals, during and just after a rainstorm and is done if faecal coliform contamination is suspected from storm drains carrying urban storm water runoff. Wet weather samples can then be compared to dry weather samples which are taken during a time of dry weather.
The bottles used for the dissolved oxygen test might also be used for the faecal coliform test. Try to avoid sampling stagnant areas of rivers. To sample, place the sample bottle under water before removing the lid.
Remove the lid and face the bottle into the current. Avoid touching the inside of the jar or lid. Place the lid back on while the jar is still in position under water. Avoid sampling the surface water because the surface film often contains greater numbers of faecal coliform bacteria than is representative of the river. Also avoid sampling the sediments for the same reason, unless this is intended.
It is a good idea to collect several samples from a particular river location to minimize the variability that comes with sampling for bacteria. Ideally, all samples should be tested within one hour of collection. If this is not possible, the sample bottles should be placed in ice and tested within six hours.
.
One way to determine the quality of lake, river or creek water is to observe what type of animals lives there. The Critter Countdown consists of a scorecard (located at the end of the page) and an identification guide. Go to a body of water and start hunting. You might want to take a net, something to use as a scope, a bucket and a magnifying lens. Use the scorecard to judge the quality of the water. Remember you may not find all the critters listed. When you are done hunting, make sure you return all the critters (unharmed) back to the water.
One Point Critters - Pollution-tolerant organisms can be in any quality of water
Aquatic Earthworms and other Worms 1/4” - 2”, can be very tiny; thin worm-like body. Tubifex worms shown.
Blackfly Larva Up to 1/4”, one end of body wider; black head, suction pad on end.
Midge Fly Larva Up to 1/4”, dark head, worm-like segmented body, 2 tiny legs on each side. Bright red or green body. Red ones also called bloodworms.
Other Snails No operculum (hard end cover), breathe air; snail shell coils in one plane
Leech 1/4” - 2”, brown, slimy body, ends with suction pads.
The long tube is used to breathe.
Rat-tailed Maggot
1/4” to 1”; the body is covered with fine hairs and is wrinkled.
Pouch Snail and Pond
Snail No operculum (hard end cover). Breathe air; shell usually opens on left.
Mosquito Larva and Pupa Fused segments makes the thorax thicker than the rest of body.
They feed on algae and other bits of organic debris. Larva (top) pupa (bottom)
Two Point Critters - Somewhat pollution tolerant organisms can be in good to fair quality water
Crayfish Up to 6”, 2 large claws, 8 legs, resembles small lobster
Damselfly Larva 1/2” - 1”, large eyes, 6 thin hooked legs, 3 broad oar-shaped tails, positioned like a tripod. Smooth (no gills) on sides of lower half of body.
Fingernail Clam Small
Crane Fly Larva 1/3” -2”, milky green or light brown, plump caterpillar-like segmented body, 4 finger-like lobes at back end.
Scud 1/4”, white to gray, body higher than it is wide, swims sideways, more than 6 legs, resembles small shrimp.
Dragon Fly Larva 1/2” -2”, large eyes, 6 hooked legs; wide oval to round abdomen.
Alderfly larva 1” long; looks like small hellgrammite but has 1 long, thin, beached tail at back end (no hooks).
No gill tufts underneath.
Fishfly larva Up to 1 1/2” long; looks like small hellgrammite, but often a lighter reddish-tan colour, or with yellowish streaks. No gill tufts underneath.
Sowbug 1/4”-3/4”, gray oblong body wider than it is high, more than 6 legs, long antennae.
Three Point Critters - Pollution sensitive organisms found in good quality water.
Stonefly Larva 1/2” - 1 1/2”, 6 legs with hooked tips, antennae, and 2 hair-like tails.
Smooth (no gills) on lower half of body.
Mayfly Larva 1/4” -1”, brown, moving, plate-like or feathery gills on sides of lower body, 6 large hooked legs, antennae, 2 or 3 long, hairlike tails. Tails may be webbed together.
Dobsonfly Larva (Hellgrammite) 3/4” - 4”, dark-coloured, 6 legs, large pinching jaws, eight pairs feelers on lower half of body with paired cotton-like gill tufts along underside, short antennae, 2 tails and 2 pairs of hooks at back end.
Caddisfly Larva Up to 1”; 6 hooked legs on upper third of body, 2 hooks at back end. May be in a stick, rock or leaf case with its head sticking out.
May have fluffy gills tufts on lower half.
Water Penny 1/4” flat saucer-shaped body with a raised bump on one side and 6 tiny legs on the other side. Immature beetle.
Gilled Snail Shell opening covered by thin plate called operculum. Shell usually opens on right.
Riffle Beetle 1/4” oval body covered with tiny hairs, 6 legs, antennae. Walks slowly underwater, does not swim on surface.
ELECTRCIAL CONDUCTIVITY
Electrical conductivity: the property of a substance which enables it to serve as a channel or medium for electricity
Salty water conducts electricity more readily than purer water. Therefore, electrical conductivity is routinely used to measure salinity. The types of salts (ions) causing the salinity usually are chlorides, sulphates, carbonates, sodium, magnesium, calcium and potassium.
While an appropriate concentration of salts is vital for aquatic plants and animals, salinity that is beyond the normal range for any species of organism will cause stress or even death to that organism. Salinity also affects the availability of nutrients to plant roots.
Depending on the type of salts present, salinity can increase water clarity. At very high concentrations, salts make water denser, causing salinity gradations within an unmixed water column and slightly increasing the depth necessary to reach the water table in groundwater bores.
Electrical conductivity in waterways is affected by:
•geology and soils
•land use, such as agriculture (irrigation), urban development (removal of vegetation, sewage and effluent discharges), industrial development (industrial discharges)
•flow (electrical conductivity is generally lowest during high flows and increases as flows decrease, with extreme levels occurring during droughts)
•run-off
•groundwater inflows
•temperature
•evaporation and dilution.
Suggested methods, equipment and reporting
The basic unit of measurement of electrical conductivity is micro Siemens per centimetre (µS/cm) or deciSiemens per meter (dS/m). Micro Siemens per centimetre are sometimes called EC units. The total dissolved solids (or TDS) content of a water sample, in milligrams per litre (mg/L), is also a measure of salinity.
The sample's electrical conductivity can be converted to TDS.
The electrical conductivity of water samples should be measured on the spot at the water body. Measurement can be delayed by up to 1 month if the sample is refrigerated (but NOT frozen) immediately on being taken, and if the sample bottle is filled completely, with no air gap at the top.
Equipment
The equipment you will need for this method includes:
•electrical conductivity meter
•calibration solution
•deionised water
An electrical conductivity meter uses two electrodes, one of which detects an electrical current sent by the other. The meter should also measure temperature and automatically compensate for temperature in the conductivity reading, but non-compensating meters do exist.
If you have a non-compensating meter, you must measure the water temperature at the same time as the electrical conductivity.
When comparing salinities of different samples, it is important to standardise the reading to 25°C. Do this by increasing the electrical conductivity reading by 2% per degree for samples with temperatures below 25°C, and decreasing it by 2% per degree for samples above 25°C.
Different meters are available for fresh water (0-1990 µS/cm), and brackish (slightly salty) water, (0-19 900
µS/cm), and sea water (~50 000 µS/cm). Use a meter that matches the expected conductivity range of your waterway.
Procedure
1. Before going to the site, calibrate your meter.
2. On site, rinse the electrode in deionised water.
3. Collect a water sample using the normal sampling technique.
4. Dip the electrode into the sample and, if appropriate, select the appropriate conductivity range.
5. Do not immerse the probe too far (some probes/meters are not waterproof above a certain point).
6. Move the electrode slowly in a circle for one minute until the digital read-out stabilises or continually jumps between two numbers.
7. Repeat the test on a replicate sample.
8. Record both results on a water quality results sheet.
9. Dispose of the sample downstream of your test site.
10. Rinse the electrode with deionised water before testing the next sample.
If the conductivity of the sample exceeds the range of the meter, you may dilute the sample. Be sure to perform the dilution according to the manufacturer's instructions because the dilution may not have a simple linear relationship to the conductivity.
Some electrical conductivity ranges Water type Electrical conductivity (µS/cm)
Deionised water
Pure rainwater
Freshwater rivers
Marginal river water
Brackish water
Saline water
0.5-3
<15
0-800
800-1600
1600-4800
>4800
Seawater
Industrial waters
51 500
100-10 000
Source: Suttar S., Ribbons of Blue Handbook. Scitech, Victoria, 1990.
Chloride and Salinity
INTRODUCTION
Chloride
Chloride, in the form of the Cl– ion, is one of the major inorganic anions, or negative ions, in saltwater and freshwater. It originates from the dissociation of salts, such as sodium chloride or calcium chloride, in water.
NaCl(s) →
CaCl2(s) →
These salts, and their resulting chloride ions, originate from natural minerals, saltwater intrusion into estuaries, and industrial pollution. There are many possible sources of manmade salts that may contribute to elevated chloride readings. Sodium chloride and calcium chloride, used to salt roads, contribute to elevated chloride levels in streams. Chlorinated drinking water and sodium chloride water softeners often increase chloride levels in wastewater of a community.
In drinking water, the salty taste produced by chloride depends upon the concentration of the chloride ion.
Water containing 250 mg/L of chloride may have a detectable salty taste if the chloride came from sodium chloride. The recommended maximum level of chloride in U.S. drinking water is 250 mg/L.
Salinity
Salinity is the total of all non-carbonate salts dissolved in water, usually expressed in parts per thousand (1 ppt
= 1000 mg/L). Unlike chloride (Cl–) concentration, you can think of salinity as a measure of the total salt concentration, comprised mostly of Na+ and Cl– ions. Even though there are smaller quantities of other ions in seawater (e.g., K+, Mg2+, or SO4 2–), sodium and chloride ions represent about 91% of all seawater ions. Salinity is an important measurement in seawater or in estuaries where freshwater from rivers and streams mixes with salty ocean water. The salinity level in seawater is fairly constant, at about 35 ppt (35,000 mg/L), while brackish estuaries may have salinity levels between 1 and 10 ppt. Since most anions in seawater or brackish water are chloride ions, salinity can be determined from chloride concentration.
The following formula is used: salinity (ppt) = 0.0018066 ✕ Cl– (mg/L)
A Chloride Ion-Selective Electrode can be used to determine the chloride concentration, which is converted to a salinity value using the above formula. Salinity can also be measured in freshwater. Compared to seawater or brackish water, freshwater has much lower levels of “salt ions” such as Na+ and Cl–; in fact, these ions are often lower in concentration than hard-water ions such calcium (Ca2+) and bicarbonate (HCO3 –). Because salinity readings in freshwater will be significantly lower than in seawater or brackish water, readings are often expressed in mg/L instead of ppt (1 ppt = 1000 mg/L).
.
Expected Levels
Seawater has a chloride ion concentration of about 19,400 mg/L (a salinity of 35.0 ppt). Brackish water in tidal estuaries may have chloride levels between 500 and 5,000 mg/L (salinity of 1 to 10 ppt). Even freshwater streams and lakes have a significant chloride level that can range from 1 to 250 mg/L (salinity of 0.001 to 0.5 ppt).
Summary of Methods
Method 1: Chloride Concentration and Salinity (ISE)
A Vernier Chloride Ion-Selective Electrode is used to measure the chloride ion concentration in the water (in mg/L) either on site or after returning to the lab. Salinity can be determined using the relationship, salinity
(ppt) = 0.0018066 ✕ Cl– (mg/L).
Method 2: Salinity Using a Conductivity Probe (Seawater or Brackish Water)
A Vernier Conductivity Probe is used to measure the salinity value of the water (in ppt). If salinity values exceed 13 ppt, dilution of samples, as described in the procedure, will be necessary. This method uses the assumption that most of the ions in the solution are noncarbonated salt ions (e.g., Na+, K+, or Cl–), and converts the conductivity reading to a salinity value.
Method 1: Chloride Concentration and Salinity (ISE)
1.
Rinse the ISE with distilled water and gently blot it dry.
2.
Place the tip of the probe into the stream at Site 1, or into a cup with sample water from the stream.
Make sure the ISE is not resting on the bottom and that the small white reference contacts are immersed. Make sure that no air bubbles are trapped below the ISE.
3.
Important: Leave the probe tip submerged for the 10 seconds that data is being collected.
4.
When the sampling run is complete, record the mean chloride concentration value.
5.
Return to Step 1 to obtain a second reading.
6.
Determine the salinity value of your sample (in ppt). Use this formula to calculate the salinity, based on the chloride concentration, Cl– salinity (ppt) = 0.0018066 X Cl– (mg/L)
7.
Record this value (round to the nearest 0.1 mg/L Cl–).
Method 2: SALINITY USING A CONDUCTIVITY PROBE
1.
Place the tip of the electrode into a cup with sample water from the body of water you are testing. The hole near the tip of the probe should be covered completely.
2.
Monitor the salinity concentration value in the meter.
3.
If the value is less than 13 ppt (the upper range of the Conductivity Probe), record this value
4.
If you did a 1/4 dilution of the sample, be sure to multiply this value by 4!
5.
If the salinity value is greater than 13 ppt, go to Step 7.
6.
Repeat Step 1 to obtain a second reading.
7.
If your salinity value is greater than 13 ppt, then the sample had a salinity value that is beyond the upper range of the Conductivity Probe. You will need to dilute the sample to a salinity value that is within the range of the probe. To do this, use a graduated cylinder to add 100 mL of the water sample to a 500 mL bottle.
8.
Add 300 mL of distilled water to the sample in the bottle, screw the lid on, and shake it well.
9.
Important: The sample has been diluted by a factor of 100/400 or ¼. Any salinity measurements made on this sample will have to be multiplied by a factor of 4 to account for this dilution.
10.
Repeat Step 7 to obtain a second reading.
Q-value
100
90
80
70
60
50
40
30
20
10
0
0
Chart 1: Dissolved Oxygen (DO) Test Results
20 40 60 80 100 120 140 160 180 200
DO: % saturation
Note: if DO % saturation > 140.0, Q=50.0
100
70
60
Q-value
50
90
80
40
30
20
10
0
2 3 4 5
Chart 3: pH Test Results
6 7 8 9 pH: units
Note: if pH<2.0, Q=0.0
if pH>12.0, Q=0.0
10 11 12
60
Q-value
50
40
30
20
10
0
0
100
90
80
70
5
Chart 4: 5-Day Biochemical Oxygen Demand (BOD
5
) Tests
10 15 20 25 30 35 40 45 50