Dissolved Oxygen
advertisement

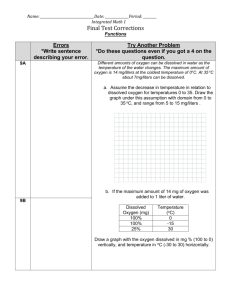
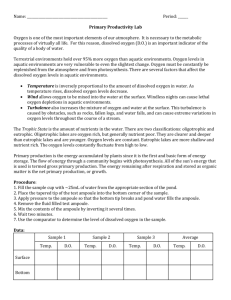
Name________________________________________________________________________Period ____ Virtual Lab: Dissolved Oxygen The dissolved oxygen concentration in a body of water is often used as a benchmark indicator of water quality and is considered a limiting factor in aquatic ecosystems. We know that oxygen is critical for the life processes of all organisms and those who live underwater are no different. Since aquatic organisms must obtain their oxygen from the water, the oxygen itself must be in a free state, dissolved in solution. Dissolved oxygen is measured in units called parts per million units (ppm). Most fish species require a minimum of 8 ppm dissolved oxygen to survive. A few highly adapted fish, such as a Betta and some catfish can survive at levels as low 3 ppm. In an environment with below 2 ppm, only benthic worms and some larval forms of invertebrates can thrive. How does oxygen become dissolved in water? Most oxygen is diffused through wind, wave action or other types of water movement. Photosynthetic organisms such as phytoplankton also contribute a large amount through photosynthesis. Physical factors such as temperature, dissolved solids and the partial pressure of the oxygen in the air itself, also influence the rate at which oxygen enters the water. In this activity, you will be determining how temperature, density and water movement affect the amount of dissolved oxygen in water. Problem: How does temperature affect the amount of dissolved oxygen in a water sample? Hypothesis: Procedure: this is a virtual lab 1. 2. 3. 4. Click the link provided on my webpage to find the lab Go through the “getting started” (video may not work) Once you enter the simlab, you can access this same procedure information in the tabs on the left side Follow the procedure. Be sure to record the starting point of the solution in the burette before you start titration each time! 5. After you have completed the basic procedure for the four samples, close the virtual lab and continue with this lab sheet. You do not need to complete the graph or the questions from on line. Data: sample 5 oC 10 oC 15 oC 20 oC Burrette start Burrette end Titrant used Dissolved O2 (mg/L) Analysis Questions: 1. Why is dissolved oxygen concentration a good indicator of water quality? 2. What must happen in aquatic environment for oxygen to be used by water dwelling organisms? 3. What units are used to measure dissolved oxygen in solution? 4. What is the minimum amount of dissolved oxygen that most fish need to survive? 5. What type of organisms typically can survive and even thrive with less than 2 ppm? 6. What are two ways that oxygen enters an aquatic environment? 7. What are three physical factors that can effect waters oxygen content? 8. What happened to the oxygen content as the temperature increased? 10. In the ocean, upwelling occurs when nutrient rich water from the bottom of the ocean is brought to the surface by rising currents. In areas where upwelling occurs, there is a huge increase and variety of animal life. Explain why you think this increase in the animals happens in these areas. In a body of water, both fresh and salt, there are typically 3 main life zones. The surface is called the photic zone and is defined as the depth at which light for photosynthesis can penetrate. Below the photic zone is the aphotic zone which does not have enough light to support photosynthesis. This zone becomes increasingly darker the deeper you go. The benthic zone is at the very bottom. Depending on depth and clarity of the water, this area could be pitch black. Though these zones vary in the amount of light they posses, each does contain organisms with special adaptations that allow them to survive in these sometimes unfriendly life zones. 11. In what zone would you find the most aquatic organisms of all types? 12. List three limiting factors in aquatic ecosystems that would affect the quantity and quality of life in the benthic zone. Explain Data Analysis & Conclusion Must be Attached ! They are worth 20 points of this 50 point lab.