To Find the Specific Latent Heat of Fusion of Ice
advertisement

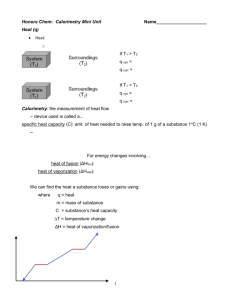
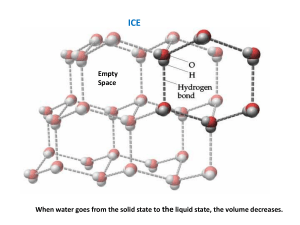
To Find the Specific Latent Heat of Fusion of Ice Equipment: Ice cubes and a clean cloth and a heavy object to crush ice. A beaker of water A low specific heat capacity thermometer (Digital with 2 places!) A copper calorimeter and insulated container with lid and stir Method: 1. Crush the ice, place it in the beaker of water and leave there for approximately 20 minutes (until it begins to melt at 0˚C). Use a thermometer to measure the temperature. 2. Find the mass of the empty calorimeter using a balance. mcal 3. Heat some water to approx. 7˚C above room temperature Fill the calorimeter about 2/3’s full with this water and find the combined mass of the calorimeter and water, then subtract to find the mass of the water and calorimeter. m2. 4. The mass of the water mw is m2 – mcal. 5. Place the calorimeter into its insulated container with a low specific heat capacity thermometer and a stir. Place the lid on top. 6. Dry some of the crushed ice and add to the water. Stir until it melts and continue to add more ice until the temperature of the water drops to 7˚C below room temperature 7. Leave for 2 minutes to ensure the temp of the water has reached its lowest, and then record the temperature. 8. Find the total mass of the calorimeter, water and ice, m3,, and then subtract m2 to find the mass of the ice added. 9. The mass of the melted ice mi is m3 – m2. 10. Use the following formula to find the specific latent heat of fusion of ice: MwCwΔθ + McCcΔθ = MiL + MiwCwΔθ Hypothetical Results: Mi = 0.0151Kg Mc = 0.0467Kg Start Temp =30.5˚C End Temp = 10.2˚C Mw = 0.0583kg Mc = 0.065KG (0.0583)(4200)(20.3) + (0.065)(390)(20.3) = (0.0151)L + (0.0151)(4200)(10.2) 5485.263 = 0.0151L + 646.884 L = 320422.4503; L = 3.2 X 105 J Kg-1 0.0151L = 4838.79 Precautions: Use a stirrer to ensure the heat energy is evenly distributed Ensure the calorimeter is insulated so that heat does not escape into the environment Use a lid to prevent the loss of heat to the environment Use a low specific heat capacity thermometer to ensure minimal energy is removed from the water in order to raise the length of the column of liquid. Use a digital thermometer correct to 2 decimal places.. Polish the inside of the calorimeter before the experiment as the shiny surface will reflect heat inwards, thus preventing heat loss to the environment. Ensure the temperature of the water begins 7˚C above room temperature and finishes 7˚C below room temperature. By starting above room temperature some heat will be lost to the environment and when the temperature drops below room temperature heat will be taken in. By starting and finishing with the same degree of difference to room temperature heat lost will balance heat gained, thus negating the effect on our results and making them more accurate. Also, because we are using warm water the ice will melt more quickly; thus the experiment will happen faster and hence less heat will be transferred in or out. Lastly, using warm water allows us to use a greater mass of ice which will reduce percentage error in reading the mass of ice. To avoid melting the crushed ice, transfer it with a plastic spatula. Results: Mass of the calorimeter mcal = Mass of the calorimeter plus water m2 = Mass of the water mw = m2 – mcal = Initial temperature of the calorimeter plus water θ1 = Final temperature of the calorimeter plus water plus melted ice θ2 = Rise in temperature of the ice = θ2 – 0 °C = θ1 Fall in temperature of the calorimeter plus water = θ1 – θ2 = θ 2 Mass of the calorimeter plus water plus melted ice m3 = Mass of the melted ice mi = m3 – m 2 = Energy gained by ice = energy lost by calorimeter + energy lost by the water. mil + micw θ1 = mcalcc θ 2 + mwcw θ 2 Notes If a polystyrene container is used in place of the copper calorimeter, the energy gained by the ice is equal to the energy lost by the water. The energy equation now reads: mil + micw θ1 = mwcw θ 2 . To avoid melting the crushed ice, transfer it with a plastic spatula. Additional Information: If you are asked to transfer hot copper into a calorimeter: Energy lost by copper rivets = energy gained by copper calorimeter + the energy gained by the water mcocc θ1 = mcalcc θ 2 + mwcw θ 2 . Ensure that the rivets are small and that you have a thermometer in the middle of them. Leave for 15 minutes after the water comes to the boil to ensure the copper is at 1000C. If you are asked the room temperature: Answer: It is half way between the initial temp and the finishing temp.