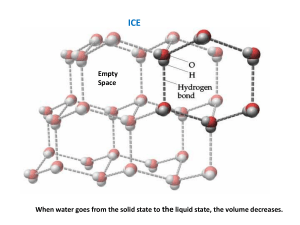
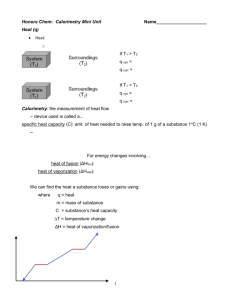
Here
advertisement

AP Chemistry Name: ___________________________ Group Members: ___________________________ ___________________________ ___________________________ Molar Enthalpy of Fusion of Ice Purpose and Objectives In this lab, the heat of fusion for water will be determined by monitoring the temperature changes while a known mass of ice melts in a cup of water. The experimentally determined value for heat of fusion will be compared with the accepted standard value. We will also explore the following question regarding the rates of melting for ice cubes is posed: Will ice cubes melt faster in distilled water or in salt water? Safety and Disposal Normal laboratory precautions, including wearing goggles and aprons at all times, should be taken to protect you at all times. Use of heated liquids can cause burns. Use hot hands to transfer hot liquids and take care when transferring liquid from one container to another. Equipment 250 mL beaker temperature probe distilled water Hot hand 100 ml graduated cylinder Scoopula Ice thermometer (optional) balance Styrofoam calorimeter Hot plate Procedure 1. Setup the LabQuest Mini with a temperature probe attached. Enter settings so that data can be collected when started. 2. Obtain bout 20 g of ice cubes and set them on a paper towel to begin melting. AP Chemistry 3. Measure the mass of the empty Styrofoam cup. 4. Measure 100 mL of warm water (50°C to 55°C) with a graduated cylinder. Pour the water into the Styrofoam cup. Find the mass of the cup and the water. Assemble calorimeter by firmly attaching the cup. 5. Insert the temperature probe into the calorimeter, making sure the probe is completely immersed in the water. 6. Begin collecting data. 7. Gently pat water from the surface of the ice. Lift the lid and carefully add the ice to the water in the calorimeter. Quickly replace the lid on the calorimeter. 8. Gently swirl the calorimeter to mix the ice-water mixture. Be certain that the probe is immersed in the ice-water. 9. Collect data until a constant temperature has been reached for about 2 minutes. 10. End data collection and view the graph. 11. Remove the probe from the calorimeter. You will need to take special care to shake water drops on the probe back in the calorimeter. 12. Find the final mass of the calorimeter with the melted ice and water. AP Chemistry Name: ___________________________ Group Members: ___________________________ ___________________________ ___________________________ MOLAR ENTHALPY OF FUSION OF ICE LAB REPORT FORM Data You will need to construct data tables to display values. Data Analysis 1. Use your data to calculate the molar enthalpy of fusion for the ice. Show calculations 2. Calculate the Percent error of your experimental molar enthalpy of fusion value using the accepted value of 6..02 kJ/mol. Show work. 3. Why is it better to put ice rather than an equal mass of cold water into a drink you want to chill? 4. Before the ice is placed in the calorimeter, the ice is dried. Why did it need to be dry? If the ice was not dry, would the experimental value of the molar enthalpy of fusion be higher or lower than the expected value? Explain. 5. If ice cubes are taken from a freezer and used immediately in the experiment, then will the value of the latent heat of fusion be less than or greater than the theoretical value? Explain. Conclusion You will need to write a proper conclusion relating what you saw in the lab and how it relates to the topics covered in class. Be specific in your relation of observations to the results. Be sure to include any possible sources of error with a discussion of how that error could have affected the results.