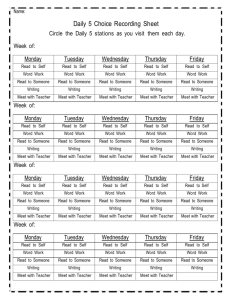
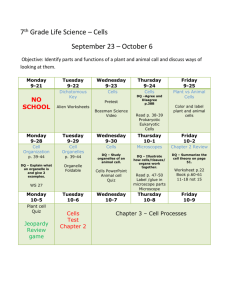
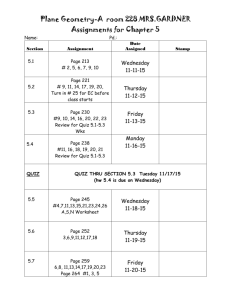
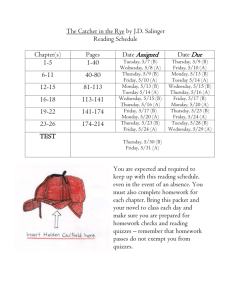
Chemistry 121: Introduction to Inorganic Chemistry
advertisement

Chemistry 121: Introduction to Inorganic Chemistry Fall 2008 Chem 121 is the first of a 2 quarter series covering the fundamental concepts of inorganic, organic, and biological chemistry and is intended primarily for students in various healthrelated programs. Previous chemistry is required, either high school chemistry or Chem 100. Chem 101 requires moderate proficiency with algebra; as such Math 95 or Math 98 is a prerequisite (but may be taken concurrently). Suggested English language skills are placement in English 101. Chem 121 will develop the central concepts of measurement and determination of physical properties; scientific and analytical methodology, the nature of atoms, molecules, and ions; bonding between atoms and molecules; mass relationships and stoichiometry; solubility and solution chemistry. Additionally, Chem 121 will focus on chemical reactivity in terms of equilibrium and kinetics. Acids, bases, and pH will be considered in some depth, and oxidation and reduction will also be developed. The course will be presented within a framework of practical relevance, and should provide a “chemical scaffold” on which to interpret physical phenomena, with examples geared towards the health science student. Instructor: Shane Hendrickson Office: Cascade Building, Room 204A, Phone: (253) 964-6236 e-mail: SHendrickson@pierce.ctc.edu Official Office hours: M-F 10 am to 11:30 am or by appointment Required Text1: General, Organic, and Biochemistry, 2nd edition, Ira Blei & George Odian, W.H. Freeman and Co. (2006). Time & Location Lecture: M, T, W from 1:00 to 2:05 pm, Rm C202 Laboratory: Th from 1:00 - 2:50 pm (section CF) or M from 2:15 – 4:05 pm (section DF), Rm C204 1 The Chem 121 lab manual will be provided to you Grading: The Grading for Chem 121 will be as follows 3 90 Point Examinations2, lowest normalized to 60 points 2 exams(90 points/exam) + 1 exam(60 points/exam) = 240 points (40.0%) Final Exam = 100 points (16.7%) 10 Laboratory Experiments: 10 Labs(10 points/Lab) = 100 points (16.7%) 3 Quizzes, lowest dropped: 2 Quizzes(30 points/Quiz) = 60 points (10.0%) 5 Homework Assignments: 5 HW(10 points/HW) = 50 points (8.3%) 5 Group Exercises: 5 GE(10 points/GE) = 50 points (8.3%) Total graded3 points = 600 Grades will be assigned as a percentage of total points possible, as follows: % >92 91.0-91.9 90.0-90.9 89.0-89.9 88.0-88.9 87.0-87.9 86.0-86.9 85.0-85.9 84.0-84.9 Grade 4.0 3.9 3.8 3.7 3.6 3.5 3.4 3.3 3.2 % 83.0-83.9 82.0-82.9 81.0-81.9 80.0-80.9 79.0-79.9 78.0-78.9 77.0-77.9 76.0-76.9 75.0-75.9 Grade 3.1 3.0 2.9 2.8 2.7 2.6 2.5 2.4 2.3 % 74.0-74.9 73.0-73.9 72.0-72.9 71.0-71.9 70.0-70.9 69.0-69.9 68.0-68.9 67.0-67.9 66.0-66.9 Grade 2.2 2.1 2.0 1.9 1.8 1.7 1.6 1.5 1.4 % 65.0-65.9 64.0-64.9 63.0-63.9 62.0-62.9 61.0-61.9 60.0-60.9 59.0-59.9 <59 Grade 1.3 1.2 1.1 1.0 0.9 0.8 0.7 0.0 As may be noted from the above, the course is not graded on a Gaussian distribution. I reserve the right to normalize the grades upward if the class average falls below a C+. However, the more likely occurrence will be the assigning of additional problems for extra credit 2 Exams will be a combination of multiple choice, short answer (generally mathematically based), and fill in the blank. Questions for the examinations will be taken from material covered in lecture (≥ 90%) and reading assignments (≤ 10%). 3 Non-extra credit Schedule of Examinations: Section I: Physical Properties of Substances, Atomic & Molecular Structure Wednesday, 10/15 Section II: Mass Relationships and Stoichiometry - Behavior of Gases - Monday, 11/03 Section III: Solution Chemistry, Reaction Rate & Equilibrium - Monday, 11/17 Section IV: Acids, Bases, Salts & Buffers; Nuclear Chemistry (Final Examination) – Wednesday, 12/10, 1:00 to 3:00 pm, Rm C202 Make-up Examinations Make-up exams will be considered only if you approach me before the scheduled exam with an exceptional, documentable excuse. Documentation is mandatory before a make-up examination will be provided. If you miss an exam and have not notified me beforehand you will receive a zero for that exam. Due to time constraints, make-up examinations will not be given for poor test performance. Excusal from Final In the event a student has only to pass the final to receive an A for the course, he or she is excused from taking the final examination. Since the total number of graded (non-extra credit) points in the course is 600, .92(600) = 552 points are required for an A. As a passing score for the final is 59, 493 points (552 – 59) accumulated prior to the final is required to have the examination waived. This corresponds to 98.6% of graded points (493/500) on all other work. All other work must be turned prior to the final to exercise this option. Behavior You are students who have elected to pursue careers in the health sciences. As such, little needs be said concerning behavior. You should be mindful of creating distractions, as they only impede your fellow students’ chances of success. Please note that questions are never considered a distraction, but must be limited to the topic at hand. As a courtesy to others, cell phones should be turned off, or switched to vibrate only in the event an emergency call is anticipated. Cheating will not be tolerated under any circumstances. My standing policy is to give a score of 0 for any exam a student is caught cheating on. As each exam is worth 20%, and the division of letter grades roughly corresponds to 10% of the total points available, cheating on an exam will cost you no less than 2 letter grades. Disabilities Students with disabilities who believe they may need academic adjustments, auxiliary aids or services to fully participate in course activities or meet course requirements are encouraged to register with the Access and Disability Services (ADS) office, room N-115 in the Sunrise building. You may also call the ADS office to make an appointment to meet with the ADS coordinator at (253) 964-6568, (253) 964-6526, or (253) 964-6527. Students requesting accommodations must obtaint an Approved Academic Adjustments, Auxilary Aids or Services form from ADS. Tentative Schedule of Lecture Topics Section I: Physical Properties of Substances, Atomic & Molecular Structure Week 1 Day Wednesday, 9/24 Thursday, 9/25 Subject Extra Credit Survey, Course Introduction Lab 1 (Section CF): Introduction to Chemical Lab Techniques Week 2 Day Monday, 9/29 Tuesday, 9/30 Wednesday, 10/01 Thursday, 10/02 Subject Composition of Matter, Physical Properties and Their Measurement (1.1-1.7) Lab 1 (Section DF): Introduction to Chemical Lab Techniques HW 1 Out Unit Conversions and Dimensional Analysis; Mass, Volume and Density (1.8, 1.9) Heat, Temperature, and Energy; Temperature Conversions Revisited (1.10, 1.11) Group Exercise 1 Lab 2 (Section CF): Separation of a Mixture into Pure Substances Week 3 Day Monday, 10/06 Tuesday, 10/07 Wednesday, 10/08 Thursday, 10/09 Subject Atomic Structure (2.1-2.5) Quiz 1 Lab 2 (Section DF): Separation of a Mixture into Pure Substances Atomic Structure and the Periodic Table (2.6-2.10) HW 1 Due, HW 2 Out Formation of Ions from Atoms, Ionic vs. Covalent Bonds (3.1, 3.2) Group Exercise 2 Lab 3 (Section CF): Physical Properties of Substances Week 4 Day Monday, 10/13 Tuesday, 10/14 Wednesday, 10/15 Thursday, 10/16 Subject Naming Ionic Compounds; Covalent Compounds & Lewis Structures (3.3-3.8) HW 2 Due, HW 3 Out Lab 3 (Section DF): Physical Properties of Substances Polarity in Covalent Compounds, 3-D Structure (3.9, 3.10) Exam I Review Examination I: Chapters 1 - 3 Lab 4 (Section CF): Mixtures & Compounds Section II: Mass Relationships and Stoichiometry, Behavior of Gases Week 5 Day Monday, 10/20 Tuesday, 10/21 Wednesday, 10/22 Thursday, 10/23 Subject Chemical and Molecular Formulas, The Mole Concept, Balancing Chemical Equations (4.1-4.6) Lab 4 (Section DF): Mixtures & Compounds Balancing Chemical Equations; Redox Reactions; Reaction Stoichiometry (4.5-4.8) Gas Laws I (5.1-5.6) Quiz II Lab 5 (Section CF): Stoichiometry Week 6 Day Monday, 10/27 Tuesday, 10/28 Wednesday, 10/29 Thursday, 10/30 Subject Gas Laws II, O2 , CO2 Transport (5.7-5.11); Lab 5 (Section DF): Stoichiometry Intermolecular Forces, States of Matter, and Phase Changes (6.1-6.5) HW 3 Due Intermolecular Forces Continued: Vaporization, Vapor Pressure, and the Structure of Solids (6.6-6.10) Group Exercise 3 Lab 6 (Section CF): Gas Laws Handout Section III: Solution Chemistry, Reaction Rate & Equilibrium Week 7 Day Monday, 11/3 Tuesday, 11/4 Wednesday, 11/5 Thursday, 11/6 Subject Examination II: Chapters 4 – 6 Lab 6 (Section DF): Gas Laws Handout Introduction to Solutions and Solubility (7.1 – 7.3) HW 4 Out Solutions vs. Suspensions, Molarity and Other Measures of Solution Concentration (7.1-7.6) Lab 7 (Section CF): Concentrations and Absorbance Week 8 Day Monday, 11/10 Tuesday, 11/11 Wednesday, 11/12 Thursday, 11/13 Subject Dilutions, Diffusion and Osmosis (7.7-7.16) Lab 7 (Section DF): Concentrations and Absorbance Veteran’s Day – NO CLASS Reaction Rates & Equilibrium (8.1-8.8) HW 4 Due Lab 8 (Section CF): Analysis of Aluminum Section IV: Acids, Bases, Salts & Buffers; Nuclear Chemistry Week 9 Day Monday, 11/17 Tuesday, 11/18 Wednesday, 11/19 Thursday, 11/20 Subject Examination III: Chapters 7-8 Lab 8 (Section DF): Analysis of Aluminum Acids and Bases I (9.1-9.3) HW 5 Out Acids and Bases II (9.4-9.6) Group Exercise 4 Lab 9 (Section CF): Unlabelled Bottles Week 10 Day Monday, 11/24 Tuesday, 11/25 Wednesday, 11/26 Thursday, 11/27 Subject Acids, Bases, and Salt Formation (9.7) Lab 9 (Section DF): Unlabelled Bottles Buffers and Titration (9.8-9.10) RPD – NO CLASS Thanksgiving Break Week 11 Day Monday, 12/1 Tuesday, 12/2 Wednesday, 12/3 Thursday, 12/4 Subject Applications of Radiation, Nuclear Energy (10.8-10.10) Lab 10 (Section DF): Acid-Base Neutralization Radiation, Common Decay Pathways (10.1-10.4) HW 5 Due Biological Effects of Radiation, Detection and Quantification of Radiation (10.5-10.7) Group Exercise 5 Lab 10 (Section CF): Acid-Base Neutralization Week 12 Day Monday, 12/8 Tuesday, 12/9 Wednesday, 12/10 Subject Quiz 3 Final Exam Review Final Examination