Carboxylic acid & derivatives
advertisement

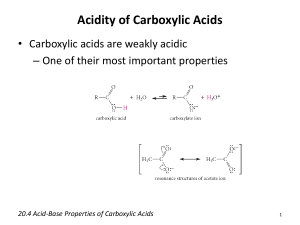
Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 1 Carboxylic Acids and their Derivatives I. Introduction Carboxylic acids are those organic compounds that contain the carboxylic group , which is the combination of the carbonyl group >C=O and the hydroxyl group –OH. O C OH Example (1) Aliphatic carboxylic acids: H H3C C CH3CH2CH2 C ________________ OH Br OH C CH2 CH2 HO OH ________________ _____________________ O O C OH _________________ Aromatic carboxylic acids: COOH COOH COOH O2N ________________ II. OH Cl OH _________________ O O CH3 CH CH2 CH CH2 C CH3 CH CH CH2 C ________________ (2) _____________________ O CH3 CH2 CH CH2 C HO OH _________________ O O C OH OH C O O O COOH _________________ _____________________ Formation of Carboxylic Acids (A) Hydrolysis of Nitriles 1. Hydrolysis of cyanohydrins or -hydroxy nitriles (prepared from aldehydes and ketones) results in -hydroxy carboxylic acids. OH R C H 2. O + HCN R C H OH H3O+ CN R C COOH H Hydrolysis of nitriles (prepared from nucleophilic substitution of alkyl halides with sodium cyanide) by a base, or by prolonged refluxing in acid solution produces the acid salt and ammonia or the acid. Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 2 Examples: NaCN BrCH2CH2CH2Br H3O+ Note: This method is limited to the use of primary alkyl halides. Use of secondary or tertiary halide often lead to elimination to form alkenes rather than substitution because the cyanide ion is a strong base. (B) Oxidation of Alkanols and Aldehydes Oxidizing agents used : KMnO4 / H+ or K2Cr2O7 / H+ + R-CHO KMnO4 / H + KMnO4 / H R-CH2OH (C) Oxidation of Aromatic side chains The side chains of alkylbenzenes are almost easily oxidized by strong oxidizing agents such as hot potassium manganate (VII) to give the acids. CH3 KMnO4 / + H HEAT Note: (1) The oxidation take place at the benzylic carbon. Alkylbenzenes with alkyl groups greater than methyl are also degraded to benzoic acid. R KMnO4 / + H HEAT (2) The oxidation begins at the benzylic hydrogen. Those do not possess any benzylic hydrogen are resistant to side chain oxidation. CH3 CH2 CH3 CH3 + KMnO4 / H HEAT NO REACTION (3) If the aromatic side chain contains an aldehyde or ketone group, the oxidation product is determined by the strength of the oxidizing agents. CH2CHO KMnO4 / HEAT CH2=CH2 + H Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 3 CH2CHO K2Cr2O7 / + H HEAT III. Acidity and Influence of substituents on acidity Carboxylic acids are rater strong acids when compared with alcohols but are weak acids when compared with mineral acids. The acid strength of carboxylic acid is sufficient enough to liberate carbon dioxide from metallic carbonate , metallic hydrogencarbonate and to liberate hydrogen from some reactive metals. CH3COOH + Na2CO3 CH3COOH + Mg Note: The acids are displaced by stronger acids from their salts. RCOO-Na+ + HCl RCOOH + NaCl Reason for acidity (A) Acidity The strength of an acid HA, i.e. the extent to which it is dissociated in the aqueous phase, is determined by the following equilibrium: H2O + HA H3O+ + A- Ka = _________________ Ka is the acidity constant. For weak acids, the value of Ka is very small. It is more convenient to express Ka by pKa where pKa = - log10Ka . The smaller the value of pKa , the ___________________ is the strength of that acid. (B) Factors affecting Acidity 1. The strength of the H-A bond. 2. The electronegativity of A 3. Factors stabilizing its conjugated anion A- with respect to HA. 4. The nature of the solvent. Example: Compare the acidity of the following compounds: OH O CH3C H A O CH3C OH B CH3CH2 OH C D Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 4 Interpretation: (1) A has the lowest acidity It is because the carbon atom which is attached to the H atom has lower electronegativity than the oxygen atom in the rest compounds. This make the O-H weaker than the C-H bond and thus it is easier to break. (2) C has lower acidity than B and D. The anion for B ( a carboxylate ion) and D (a phenoxide ion) are more stable than that of C (an alkoxide ion ) due to the stabilization of the resonance effect. (aromaticity) OH + H2O O CH3C OH these overall + H2O (3) B has higher acidity than D It is due to the fact that delocalization of the negative charge in the carboxylate anion involves resonance structures of identical energy content and involves two highly electronegative oxygen atoms. In the phenoxide anion, the resonace structure involving negative charge on the nuclear carbon atoms are likely to be of higher energy content than it is on oxygen atoms, canonical forms are therefore less stable and have a small contribution to the hybrid. (C) Effects of Substituents on Acidity can The ease of dissociation of the acidic compound, and the stability of the conjugate anion, are both affected by the substituent groups in the molecules. Any groups which can stabilize the resulting anion of the acid molecule will increase its acidity whereas any groups which destabilized the anion will also decrease its acidity. General speaking, Electron withdrawing groups increase the acidity : -F , -Br , -Cl , -NO2 Electron donating groups decrease the acidity: -CH3 , -C2H5 For carboxylic acids, <1> With electron-donating substituent O E C OH when dissociation. O E C O electron donating substituent The electron donating substituent pushes electrons towards the elecron-rich –COO- group and thus destabilize the anion. Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives <2> With electron-withdrawing substituent (E : electron withdrawing substituent) O O E C O E C OH O-H bond weakened by electron withdrawing effect of E + 5 + H anion stabilized by electron withdrawing effect of E These effect. can be seen in the following table. Interpretation <1> The more numerous the electron-withdrawing groups on the carbon, the stronger is the acid. Thus the order of acidity ethanoic acid < chloroethanoic acid < dichloroethanoic acid <trichloroethanoic acid <2> The more the electronegative the -substituent , the stronger is the acid. Thus the order of acidity ethanoic acid < iodoethanoic acid < bromoethanoic acid < chloroethanoic acid < fluroethanoic acid <3> The shorter the carbon chain on the carboxylic acid, the stronger is the acid. Thus the order of acidity Propanoic acid < ethanoic acid < methanoic acid Remark : The farther away of the electron-withdrawing substituent from the carbonyl group, the less effect on acidity. O CH3CH2CH C OH Cl Ka: 1.4 x 10-3 O O CH3CH CH2C OH Cl 8.9 x 10-5 CH2CH2CH2C OH O CH3CH2CH2C OH Cl 3.0 x 10-5 1.5 x 10-5 Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives IV. 1. 6 Reaction of Carboxylic Acids Decarboxylation This is the removal of carbon dioxide from a carboxylic acid group. The usual method is to heat the sodium salt of the acid with soda-lime (NaOH + CaO). The product is a hydrocarbon, e.g. CH3COO-Na+ + NaOH COO-Na+ + 2. NaOH Reduction Carboxylic acids are resistant to the ordinary reducing agents but lithium aluminium hydride reduces them directly to primary alcohols: LiAlH4 RCOO-Na+ / dry ether H3O+ The reagent is highly selective and the C=C , CC , or C6H5- in unsaturated acids are unaffected by it. 3. Conversion to Acid Derivatives O (i) Conversion to Acid chlorides ( R C Cl ) There are three reagents (PCl5, PCl3 and SOCl2) for the preparation of acid chlorides and all of them give high yield. O RCOOH + (1) (ii) (2) RCOOH + (3) RCOOH + R C Cl + PCl5 POCl3 + HCl PCl3 SOCl2 Conversion of Acid anhydrides Carboxylic acids react with alkanoyl chlorides in the presence of pyridine to give acid anhydrides. O RCOOH + Pyridine R C Cl What is the use of Pyridine? The pyridine is used to remove the hydrogen chloride HCl formed, so as to shift the equilibrium towards the products side. Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives (iii) 7 Conversion of Acid amides Carboxylic acids react with aqueous ammonia to form ammonium salts. By heating this ammonium salt, primary amides will be obtained. excess RCOOH RCOOH (iv) + NH3 RCOO-NH4+ RCONH2 + H2O Conversion to Ester Carboxylic acids react with alcohols in the presence of an acid catalyst to form esters through a condensation reaction known as esterification. The reaction is a reversible equilibrium process and it is acid catalysed. Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives V. 8 Acid Derivatives (A) Acid Chlorides Acid chlorides are also called alkanoyl or acyl chlorides. They are derived from the-COOH group of carboxylic acids by replacing the -OH group with -Cl group, e.g. ___________________ ____________________ ______________________ Reactions of Acid Chlorides The halogen readily undergoes nucleophilic substitution by other nucleophiles such as OH-, OR' , NH2- etc. The mechanism is quite similar to condensation reaction of aldehydes and ketones. Aromatic alkanoyl chloride like benzoyl chloride is much less reactive, due to the decrease in the nucleophilicity of the carbonyl carbon caused by resonance. 1. Hydrolysis The lower aliphatic alkanoyl chlorides are hydrolysed rapidly by cold water, e.g. CH3COCl + H2O CH3COOH + HCl When the stopper of a bottle of ethanoyl chloride is removed, white fumes are evolved, owing to interaction of the hydrogen chloride with moist air. Beware of damage to the naked eyes. Aromatic alkanoyl chlorides hydrolyse much more slowly, owing to their lower solubility and their carbonyl carbon atom being less susceptible to nucleophilic attack. The hydrolysis is faster with alkali because - OH is a stronger nucleophile than H2O. 2. Alcoholysis - Ester Formation Acid halides react with alcohols and phenols to form esters, e.g. Phenol behaves similarly as aliphatic alcohols but a base catalyst is required for it to react with aromatic alkanoyl chlorides. The basic medium provides a more powerful O nucleophilic ion, 3. (phenoxide ion) Ammolysis – Amide formation O O CH3 + C NH3 CH3 + C NH4Cl NH2 Cl Ethanamide O O C + CH3NH2 Cl C + HCl NHCH3 Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 4. 9 Anhydride Formation O O O R + C R'COONa R R' O Cl 5. C C + NaCl Reduction to Alcohols O R + C Pt + RCH2OH H2 H2 Cl 6. Ketone formation Friedel-crafts acylation O O R catalyst Cl (B) R AlCl3 + C C Acid Anhydride O Acid anhydrides are derived from carboxylic acids by replacing the -OH group in C OH by O a R C O group, e.g. _______________________ ______________________ Reactions of Acid Anhydrides Reactions of acid anhydrides are quite similar to that of acid chlorides, although their reactivity is less than that of acid chlorides. . 1. Hydrolysis Acid anhydrides are slowly hydrolysed by water to give carboxylic acids. (RCO)2O+ H2O RCOOH 2. Ester FormationAcid anhydrides react with alcohols and phenols to give esters, heating is usually required for the reaction. (RCO)2O + R'OH RCOOR' + RCOOH With phenols, alkaline medium is required to provide a powerful nucleophile, the phenoxide ion. Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 10 3. Amide Formation Acid anhydrides react with ammonia solution and amines to give amides. (RCO)2O + 2NH3 RCONH2 + RCOO-NH4+ 4. Friedel-crafts Acylation Acid anhydrides react with benzene in the presence of a little Lewis acid catalyst, AlCl3, to form a ketone. (C) ESTERS O Esters are derived from carboxylic acids by replacing the -OH group in group from an alcohol, e.g. ___________________ ____________________ C OH by an -OR ____________________ Reactions of Esters 1. Hydrolysis of Ester Acid-catalysed hydrolysis is the reverse of esterification: O R O H3O+ / Reflux C OR' + H2O R C OH + R'OH A large excess of water is used but since the reaction is reversible, the hydrolysis never goes to completion. Alkali-catalysed hydrolysis is also reversible: O R O OH- / reflux C OR' + H2O R C OH + R'OH However, in this case the carboxylic acid formed immediately reacts with hydroxide ion to give the acid anion: O O + R C OH OH- R C O + H2O The equilibrium in this step lies almost completely to the right, so that once the acid is formed in the first reaction it is immediately removed by the second reaction, until practically all the ester is converted into its hydrolysis product. Alkaline hydrolysis is therefore much faster than acid catalysed hydrolysis; and it goes to completion. The process is known as saponification 皂化反應 as soaps are made by this reaction. The resulting carboxylate product can be re-acidified to get back the carboxylic acid. 2. Ammonolysis - Amide Formation Ester reacts with ammonia or amines to form amides. Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 3. 11 Reduction to Alcohols This is a good way to prepare primary alcohols as esters are reduced by lithium aluminium hydride (LiAlH4), e.g., (D) ACID AMIDES O C OH and Amides are derived from carboxylic acids by dropping the -OH group in replacing it with a -NH, group, e.g. ___________________ _____________________ _____________________ Substitutions of the hydrogens on the nitrogen atom by alkyl groups can also occur, e.g. ________________________ __________________________ Reactions of Acid Amides 1. Hydrolysis The amides are readily hydrolysed on refluxing with dilute acid or alkali. O R O H3O+ / Reflux C NH2 + H2O R O R C C OH + NH4+ O NH2 + OH- R C O + NH3 The fact that heating an amide with caustic soda liberates ammonia whilst an amine does not react enables the two types of compound to be distinguished. 2. Dehydration Nitrile Formation Heating amides with a dehydrating agent such as phosphorus pentoxide leads to formation of nitrile. Lok Sin Tong Leung Chik Wai Memorial School F.7 Chemistry Carboxylic acids & their derivatives 3. 12 Hofmann Degradation Amides react with KOH solution and Br2 in a rearrangement reaction to give primary amines containing one carbon atom less. Thus it is useful for descending a homologous series. O CH3CH2 3. C NH2 + KOH + Br2 CH3CH2NH2 K2CO3 + KBr + H2O Reduction of Amines Amides are reduced to primary amines by either lithium aluminium hydride or sodium in ethanol (dissolving metal reduction). O R C LiAlH4 / dry ether NH2