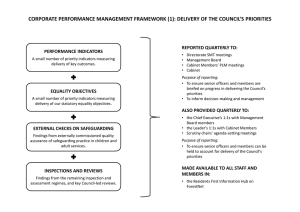
Tissue Culture Procedures - The Hospital for Sick Children

Working in the Tissue Culture Room
You must wear appropriate protective equipment and clothing such as:
Gloves…that must be replaced after 20 minutes or if they are damaged
Double gloves when working with viruses
Lab coat or protective gown
Transmission of virus can occur by: a.
direct inoculation through sharp object injury such as a Pasteur pipette;
b. contamination of damaged skin (if gloves are not worn) or;
c. spraying/splattering to mucous membranes.
NOTE: Transmission of Hepatitis B can take place through contaminated blood products. It is important to get immunized!
Start up Procedure
Turn on UV light 15 minutes prior to using biosafety cabinet. (for airborne contaminants)
Turn off UV
Turn on task lights.
Put sash in operating position (not more than a 9” opening)
Check air intake and exhaust grilles to ensure they are unobstructed
Turn on fan and allow the cabinet to run for 3-5 minutes before starting
Spray down interior surfaces with 70% ETOH. Wipe down with paper towel while still wet
Spray all materials to be transferred inside cabinet with 70% ETOH. Place a milkshake container in the hood for glass Pasteur pipettes, and for plastic pipettes intended for viral work.
While Working in Biosafety Cabinet
To avoid spills and accidents do not clutter the working area
Arrange the hood into a “clean” and “dirty” side
Handle potential biohazardous materials with care.
Practice aseptic technique to avoid contaminating your culture and yourself
(sterility is of the utmost importance). If pipettes or tips have touched any surface other than the culture, discard and use a fresh one. Plate and bottle caps should be placed face down on a clean surface if required. Cap tubes and bottles tightly when finished, and remove from cabinet. Media must be aspirated from plates using a Pasteur pipette attached to a vacuum hose and appropriate waste container. (See ‘waste disposal’ for proper disposal protocols).
Waste Disposal
All sharps such as Pasteur pipettes, and tips should be discarded into a milkshake container kept inside the cabinet.
All fluid should be aspirated from plates, flasks, or tubes unless they can be securely capped. The glass bottle on the floor used to hold liquid waste should contain bleach before you start. Add 50-100 ml of Javex before you begin. Solid waste should be discarded into the orange biohazard bags, which will be removed and autoclaved by glasswashing personnel.
Aspirated liquid in glass bottle will be removed and discarded by glasswashing personnel. However, if bottle appears full before it is cleaned add bleach, let stand 15 minutes and discard down the sink.
Note : any material which came into contact with virus must also be discarded into an autoclave bag, securely taped and dumped into the biohazard disposal green garbage bin.
Spills
Keep materials for clean up on hand. i.e. 70% ETOH, paper towels, autoclave bags, etc.
Clean up spill immediately inside the cabinet by aspirating fluid, spraying area with 70% ETOH and then soaking up all liquid with paper towel or absorbent pads. Spills outside the cabinet should be soaked in 70% Ethanol or bleach prior to wiping up.
Contaminated items should be disposed in orange biohazard bags to be autoclaved.
Finishing Procedure
After every use, all waste must be properly disposed. The glass Pasteur pipettes must be covered with a second milkshake container, sealed with autoclave tape (place in orange bag) and placed in biohazard disposal green garbage bin. Plastic pipettes and solid waste must be discarded into biohazard waste basket Plastic pipettes or waste which were in contact with virus should be discarded into an orange biohazard bag, taped up with autoclave tape and double bagged. Place bag into the green bin for biohazardous waste disposal.
Remove containers and flasks from cabinet
Clean aspiration tube by running bleach through it. (While vacuum is still on dip the tubing into a container with bleach)
Spray surface with 70% ETOH and wipe down. Pull down sash; turn off fan and lights.
Turn on UV for 15 min.
Turn Off UV
ACCIDENTS WILL HAPPEN. ALWAYS KEEP AN EMERGENCY
RESPONSE PLAN IN MIND AND IMMEDIATELY REPORT ALL
MISHAPS TO JAMES ELLIS, PETER PASCERI, AND
OCCUPATIONAL HEALTH (EXT.5997)
If you are spiked with a sharp during working hours, immediately go to the clinic on the fifth floor to be examined. After hours let affected area bleed by squeezing and then wipe with 70% ethanol. Put on Band-Aid and get checked out as soon as possible. If it is a serious emergency, then get checked out at Toronto General Hospital (Emergency Room).
Centrifugation
All centrifugation must be carried out using tightly closed containers/tubes.
Sealed cups must be used whenever hazardous materials are centrifuged. If a spill occurs, decontaminate the centrifuge immediately by removing cups, spraying down with 70% ETOH and wiping the area dry.
Incubator
Loosen all flask caps inside the incubator to allow gaseous exchange to take place (flasks with a filter top should be closed tight). Avoid any liquid inside the cap or directly inside the mouth of the flask as this may lead to contamination. Liquid inside mouth of flask can be removed by aspiration in the hood.
Keep incubator tidy and clean. Make sure water tray does not evaporate and is clean. If you see white growth, pour out the water, spray with 70%
ETOH and wipe clean. Fill tray with autoclaved water.
If a contamination occurs, remove everything from incubator. Take out all trays, and removable parts. Leave for washing personnel to wash and autoclave. Shut off the incubator and properly disinfect the interior surface with an appropriate disinfectant, then wipe down with water and finish by spraying down with 70% ETOH.
I _________________, have read and understood the tissue culture procedures.
I agree to abide by the rules outlined in the previous pages.
Trained by _____________
Signature of trainee ___________
Date __________
Signature of PI ______________
TISSUE CULTURE SUPPLIES
Medium (Gibco) Cat. #
D-MEM alpha-MEM
RPMI
Opti-MEM
11965-092
12571-063
11875-093
31985-070
Order From GIBCO
Fetal Bovine Serum (certified grade) 16000-044 heat inactivate for 30 minutes at 56 C (add 50 ml into 500 ml medium)
Trypsin-EDTA (0.25%) use as is on cells
25200-056
Geneticin (G418) (powder to be filter sterilized after made) use at 500 ug/ml final concentration ( titrate for particular cell line)
D-PBS (Gibco) 14040-133
Pen-Strep-Glut mix 10378-016 use 1 ml in 500 ml of medium (1%)
Polybrene (Hexadimethrine Bromide) ( Sigma ) H 9268 use 8 ug/ml final concentration
NUNC PRODUCTS
Tissue culture flasks
75 cm
25 cm
Cat. #
156499
Tissue culture plates
100 mm X 20
60 mm X 15
172958
150288
6-well plate 152795
Where Tissue Culture Supplies are to be Stored
Item Storage
Medium
Trypsin
Fetal Bovine Serum
Pen-Strep-Glut mix
Geniticin (G418)
Polybrene
PBS
70% ETOH
Sterile EDTA in lab fridge unit 5 stock bottles in tissue culture cold room opened bottle in lab fridge unit 5, stock kept in -20 chest freezer in lab stored in -20 chest freezer in lab stored in -20 chest freezer in lab fridge unit 5 in lab fridge unit 5 in lab fridge unit 5 in lab, and cold room on shelf next to the safety cabinet in
TC room
0.1% Gelatin
DMSO
Important Note concerning reordering items for tissue culture:
YOU NEED TO ORDER
If there is only one box of TC flasks in the lab and one in use.
If there are only 5 bottles of D-MEM left in the cold room or 2 bottles of other media
If G418 stock is getting low
If there is only one bottle of Trypsin
If you have aliquoted the last bottle of Fetal Bovine Serum
If there is only a few aliquotes of Pen-Strep-Glut Mix left
If you notice any other supplies are low in stock, such as TC plates, autoclaved tips, autoclaved Pasteur pipettes, 70% ETOH, etc. Either write it up on the board to get made/ordered or take the liberty to do it yourself .