ESPM 220c Homework 5: Hydrological Cycle
advertisement

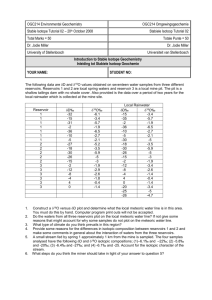
ESPM 220c Homework 2: Hydrological Cycle Name:__________________ 1. The isotopic composition of precipitation in the Mojave Desert of southern California and Nevada shows a consistent decrease in delta values with increasing elevation (Friedman et al. 1992. Stable isotope composition of waters in southeastern California. 1. Modern precipitation. Journal of Geophysical Research). Though several explanations might be applicable, one likely source of the variation (based on observation) is that there is an increasing amount of evaporation of precipitation, before it hits the ground, with decreasing elevation. Delta D (per mil) FRIEDMAN ET AL 1992 -90 y = -60.724 - 0.010262x R= 0.68288 -85 -80 Delta D (per mil) -75 -70 -65 -60 -55 -50 -500 0 500 1000 1500 2000 2500 3000 elevation (m) In the 1980’s, I worked with colleagues on the soil isotopic behavior along an elevation transect north of Las Vegas. Below are the site names (dominant vegetation) and geographical information. a. Calculate the 18O value of the precipitation at each site using the relationship that Friedman et al found for the Mojave: D = (18O•6.5)-9.7. b. Is the slope the same as the Meteoric Water Line? If not, what is the common explanation given the different slope? c. Assuming that the precipitation evaporates into a dry atmosphere, calculate the fraction of water (using O isotopes) that must have evaporated to produce the precipitation at each site, assuming the highest elevation has an f =1. 2. Using the water and temperature information, calculate the 18O value of soil carbonate that would form at each site. Does this O-bearing compound in equilibrium with water actually show the same O isotope trend with elevation (and temperature) as the rainfall? WHY (plot data to visualize trends). 3. We measured the C and O isotope composition of soil CO2 and found a consistent trend in the 18O of the with elevation that followed the trend of precipitation. A result of several years of work revealed that soil CO2 at any depth is nearly (but not quite) in isotopic equilibrium with the water at that depth (the non-equilibrium is due to the fact CO2 is constantly diffusing upward, and must equilibrate with the new water it encounters). For the data you have, calculate the 18O value of soil CO2 in isotopic equilibrium with the precipitation at the site. KYLE CANYON DATA SITE Creosote Blackbrush Pinyon-Juniper Fir-Pine PRECIPITATION CARBONATECO ELEV (M)MAT ( C)MAP (mm)D (o/oo) 18O (o/oo)18O (o/oo)18 840 17.9 160-69.34408 1400 14.1 326 -75.0908 1750 11.6 436 -78.6825 2150 9 549 -82.7873 4. The measurement of O isotopes in rain (or any water) almost always involves the equilibration of CO2 with the water in the lab, and the subsequent isotopic analysis of the isotopic composition of the CO2 (and a back calculation to what the water value is). In practice, a “small” amount of CO2 is equilibrated with a “large” amount of water – so that the water value doesn’t change and water dictates CO2 values. As an example, 10 ml of water is equilibrated with CO2 at a pressure of 1 atm in a vial that is 30 ml. Assume, for simplicity, all the CO2 is in the gas phase. a. How many moles of O are in the water? b. How many moles of O are in the CO2 in the headspace? (see Hints below). c. The equilibration takes place @ 25 0C. The 18O value of the initial CO2 is + 4 o/oo (SMOW). The 18O value of the water is – 9 o/oo. What is the equilibrium value of the CO2 ? d. In an upcoming homework, we will evaluate how FAST this reaction occurs….. HINTS: 1. Use the Rayleigh distillation model into dry air for question 1. This is reasonable given the very low relative humidities in the desert. 2. Use/familize yourself with fractionation factor equations either via the book form (Friedman and O’Neil) or the web site I provide for you. Please use (if you follow Freidman and O’Neil): a. CaCO3-water fractionation of Oneil, Clayton and Mayeda (1969) b. Carbon dioxide-water fractionation of O”Neil and Adami (1969) 3. For question 5, use the exceedingly useful relationship for gas source isotope geochemists: PV = nRT. The CRC Handbook of Chemistry and Physics (now online) has one page devoted to this important relationship and the units that can be used (moles, atm, K here).