DETERMINING AGE
advertisement

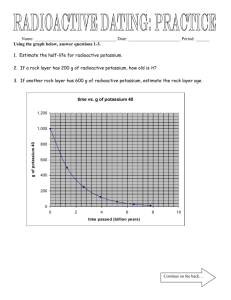
Radiometric Dating of M&Mium or Skittilium Background Information: THE AGE of fossils intrigues almost everyone. Students not only want to know how old a fossil is, but they want to know how that age was determined. Some very straightforward principles are used to determine the age of fossils. Students should be able to understand the principles and have that as a background so that age determinations by paleontologists and geologists don't seem like black magic. There are two types of age determinations. Geologists in the late 18th and early 19th century studied rock layers and the fossils in them to determine relative age. William Smith was one of the most important scientists from this time who helped to develop knowledge of the succession of different fossils by studying their distribution through the sequence of sedimentary rocks in southern England. It wasn't until well into the 20th century that enough information had accumulated about the rate of radioactive decay that the age of rocks and fossils in number of years could be determined through radiometric age dating. Most geological processes occur at an irregular and unpredictable pace. In processes such as erosion, deposition, land uplift and volcanic eruption, periods of activity occur in spurts that are separated by long periods of inactivity. Although geological processes often reveal relative time, they do not indicate absolute time. For example, we can look at a rock formation and determine which layer formed earlier and which formed later, but we cannot tell exactly how many years ago a particular layer formed. Testing radioactive minerals in rocks best determines absolute time. Radioactive decay goes on like clockwork, at an even and continuous pace. The nuclei of radioactive atoms break down, releasing particles and radiation. Finally, the radioactive element changes to a stable new element. The radioactive element is called the parent, and the stable new element is called the daughter. The rate of radioactive decay is measured by half-life - the time it takes for half of the atoms of a parent element to change into atoms of the daughter element. Consider the element radium-226, which has a half-life of 1,622 years. What happens to 10 grams of radium after 1,622 years? Five grams of radium remain, and five grams will have changed into lead. Some elements have forms (called isotopes) with unstable atomic nuclei that have a tendency to change, or decay. For example, U-235 is an unstable isotope of uranium that has 92 protons and 143 neutrons in the nucleus of each atom. Through a series of changes within the nucleus, it emits several particles, ending up with 82 protons and 125 neutrons. This is a stable condition, and there are no more changes in the atomic nucleus. A nucleus with that number of protons is called lead (chemical symbol Pb). The protons (82) and neutrons (125) total 207. This particular form (isotope) of lead is called Pb-207. U-235 is the parent isotope of Pb-207, which is the daughter isotope. Very careful measurements in laboratories, made on VERY LARGE numbers of U-235 atoms, have shown that each of the atoms has a 50:50 chance of decaying during about 704,000,000 years. In other words, during 704 million years, half the U-235 atoms that existed at the beginning of that time will decay to Pb-207. This is known as the half life of U- 235. Each unstable isotope has its own characteristic half life. Some half lives are several billion years long, and others are as short as a ten-thousandth of a second. Many rocks contain small amounts of unstable isotopes and the daughter isotopes into which they decay. Where the amounts of parent and daughter isotopes can be accurately measured, the ratio can be used to determine how old the rock is, as shown in the following activity. A tasty way for you to model and understand half life is to use M & Ms or Skittles candy. Objective: Students will become familiar with the concept of half-life in radioactive decay. Students will see that individual runs of statistical processes are less predictable than the average of many runs Students will demonstrate how the rate of radioactive decay of the parent and the buildup of the resulting daughter is used in radiometric dating of rocks. Students will model radiometric dating and the principles of determining relative age to show how ages of rocks and fossils can be narrowed even if they cannot be dated radio metrically. Required Materials 1. 2. 3. 4. 5. Large flat container with lid 100 M & M's / Skittles (don’t eat ANY until the end of the lab!) Data table (on next page) Stopwatch Graph paper In this lab, you will experiment with a half-life model in which M&M candies or Skittles represent radioactive atoms. The imprinted "M" or “S” on each candy represents the stable daughter element resulting from radioactive decay. Students place the candies label-side down in a box, shake them, and then count the number of "changed" atoms. Predict: Predict how many trials you will go through before the candies have all “turned” into the daughter isotope. This will be your hypothesis. Procedure WASH your hands! 1. Place the candies "M" or “S” -side down in the box. 2. Close the cover and shake for 10 seconds. (use timer) 3. Open the box and remove all the "changed" candies (those turned "M" or “S” -side up). 4. Count and record the number of "unchanged" candies remaining in the box. Record this data on a chart similar to the one below, or of your own design. Make sure you give yourself enough rows, as you can’t be sure how many trials it will take to run out of your radioisotopes M&Mium and Skittilium. 5. Repeat steps 1-4 until all the candies have turned. 6. Graph your data from the lab. On the graph, draw a curve in red for the data. In this model of half-life decay, each shake is comparable to the passing of time: the number of "unchanged" candies is comparable to the number of unchanged atoms. Then, in blue, plot the points of the evolving daughter isotope, drawing the curve for that as well. USE your graphing skills! Titles, labeled axes, and some sort of key. HalfLife 1 2 3 4 5 6 7 8 9 10 11 Time in seconds 0 10 20 30 40 50 60 70 80 90 100 # Parent Isotope Left % Parent Isotope # Daughter Isotope Left % Daughter Isotope After the results of the final "half life" of the M& Ms and Skittles are collected, the candies are no longer needed. You many then consume them! Thus, the importance of washing your hands! Analysis Questions: 1) Why didn't each group get the same results? 2) Look at the data in your table at 3 half-lives. Pretend your rock specimen was found at this point and analyzed. If the half-life of this candy is 2,500,000 years, what was the age of your specimen at this point? 3) What percentage of parent material was left after 8 half-lives? 4) Name three typical forms of radiometric dating that you have learned about. 5) Describe one thing that you know now about radiometric dating that you did not know before this lab.