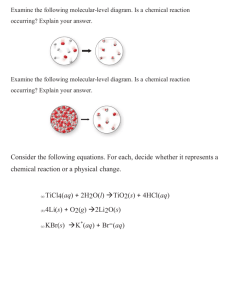
Example: A Chemical Reaction
advertisement

1 CHEMICAL REACTIONS Example: Hydrogen + Oxygen Water H2 + O2 H2O reactants product(s) reactant – substance before chemical change product – substance after chemical change Conservation of Mass During a chemical reaction, matter is neither created nor destroyed. - number of atoms of each element is same before, during and after reaction. Reconsider above example + + - Note there is not enough hydrogen to react with oxygen - It is necessary to “balance” equation. 2 H2 + + O2 - This is an example of a balanced chemical reaction. BALANCING CHEMICAL EQUATIONS - ensures # of reactant atoms = # of product atoms C2H4 + O2 CO2 + H2O Both sides have 2 C atoms, 4 H atoms and 6 O atoms. Cu + S8 Cu2S Fe2O3 + C Fe + Sc2O3 + H2O Sc(OH)3 CH3NH2 + O2 CO2 + CO2 H2O + N2 2 H2O (balanced equation) 2 - Sometimes it is more convenient to balance groups of atoms than individual atoms. - i. e., balance polyatomic ions Pb(NO3)2 KBr + KNO3 + PbBr2 - Balance NO3- ions rather than N and O atoms. Ca(ClO3)2 Fe2(SO4)3 + Fe(ClO3)3 + CaSO4 Cu(OH)2 + NaC2H3O2 - Balance ClO3- and SO42- ions Cu(C2H3O2)2 + NaOH AgNO3 + CaCl2 Ca(NO3)2 + Ba(ClO4)2 + Na2SO4 BaSO4 + NaClO4 AgCl IONIC EQUATIONS AND THE AQUEOUS PHASE Aqueous Phase Q: What happens to salt when it is dissolved in water? - It is a physical change but, how can that be? A: Solid salt is a collection of ions, Na+ and Cl-, assembled in a lattice - Ions are surrounded by each other. When dissolved, the ions are surrounded by water - Thus, the ions themselves don’t change, only their environment. + - + - - + - + + - + - H H O H H O + O H H - + - O + H ion surrounded by other ions H ion surrounded by water molecules (solvent cage) Ions (or sometimes molecules) that are surrounded by water are in the aqueous phase. 3 Ionic compounds which are soluble (that is, dissolve in water) dissociate (that is, break apart) into dissolved cations and anions. Examples: NaCl (aq) = Na+ (aq) + Cl- (aq) K2O (aq) = 2 K+ (aq) + O2- (aq) - note 2 K+ ions dissociate for every K2O unit. Ca(NO3)2 (aq) = Ca2+ (aq) + 2 NO3- (aq) - ions dissociate, but polyatomic ions remain covalently bonded. *Not all compounds dissociate into ions when in the aqueous phase, but we will ignore this temporarily.* Aside: phases of reactants and products (g) – gas (l) – liquid (s) – solid (aq) – aqueous IONIC EQUATIONS - molecular equations where all ions in aqueous solution are written in dissociated form NET IONIC EQUATIONS - ionic equations where spectator ions (ions unchanged in reaction) are not included Example: Molecular Equation 2 KBr (aq) + Pb(NO3)2 (aq) 2 KNO3 (aq) + PbBr2 (s) Ionic Equation 2 K+ (aq) + 2 Br- (aq) + Pb2+ (aq) + 2 NO3- (aq) 2 K+ (aq) + 2 NO3- (aq) + PbBr2 (s) Net Ionic Equation Pb2+ (aq) + 2 Br- (aq) PbBr2 (s) Note removing spectator ions clarifies important part of reaction. Example: Molecular Equation HClO4 (aq) + NaOH (aq) H2O (l) + NaClO4 (aq) Ionic Equation H+ (aq) + ClO4- (aq) + Na+ (aq) + OH- (aq) Net Ionic Equation H2O (l) + Na+ (aq) + ClO4- (aq) 4 Example: Molecular Equation Fe (s) + CuSO4 (aq) FeSO4 (aq) + Cu (s) Ionic Equation Net Ionic Equation GENERAL TYPES OF REACTIONS 1) Combination - two or more substances react two form a single substance Generic reaction: A + B C a) formation reactions - elements compound 4 Fe (s) + 3 O2 (g) 2 Fe2O3 (s) The rusting of iron 8 C (s) + 9 H2 (g) C8H18 (l) The formation of octane, i.e., gasoline theoretically! b) hydration reactions - addition of water CaO (s) + H2O (l) Ca(OH)2 (s) CaO (lime) when heated emits a very bright white light (limelight). Ca(OH)2 (hydrated lime) is added to plaster to make it harder. P2O5 (s) + 3 H2O (l) 2 H3PO4 (aq) P2O5 is a very effective dehydration agent. 2) Combustion - combination with O2 (as in burning fuel) - products are CO2, H2O and N2 2 C8H18 (l) + 25 O2 (g) 16 CO2 (g) + 18 H2O (g) 2,2,4-trimethylpentane (isooctane) is the major component of gasoline. 4 CH3NO2 (l) + 7 O2 (g) 4 CO2 (g) + 6 H2O (g) + 2 N2 (g) Aminomethane is used as the fuel in top fuel dragsters. 5 3) Decomposition - one substance decomposing into two or more substances Generic reaction: A B + C Examples: CaCO3 (s) CaO (s) + CO2 (g) Calcium carbonate is the major component of seashells and egg shells. 2 NaClO3 (s) 2 NaCl (s) + 3 O2 (g) Sodium chlorate is used in the manufacture of bleach (sodium hypochlorite, NaClO). 4) Single replacement reactions - when a metal ion in a compound is replaced with another metal ion - some metal atoms are more willing to give away electrons than other metal atoms - the willingness of a metal atom to give away its electrons is called its activity Example SnSO4 (aq) + Ni (s) NiSO4 (aq) + Sn (s) Sn2+ (aq) + Ni (s) Ni2+ (aq) + Sn (s) - Ni replaces Sn2+ - Ni is more active than Sn Does the reverse reaction occur? NiSO4 (aq) + Sn (s) SnSO4 (aq) + Ni (s) NO!! - Sn is not more active than Ni Tin bonds with iron very well and helps prevent the corrosion of iron. “Tin cans” are steel(iron) with thin covering of tin. Predicting Spontaneous Single Replacement Reactions - In a reaction between metal ion (in aqueous phase) and metal, if metal is more active than metal ion, reaction will proceed. - We need to have an activity series to assess relative activity of metals. Abbreviated Activity Series Na Al Zn Cr Fe Ni Sn Pb H Cu Ag Au Increasing activity (willingness to be ionized) 6 Example: Does the reaction, Pb(NO3)2 (aq) + Cu(s) Pb (s) + Cu(NO3)2 occur? Example: Does the reaction, FeO (s) + Zn (s) Fe (s) + ZnO (s) occur? - reactant metals are Fe2+ and Zn Because zinc is more active than iron, it is used to protect iron from rusting (galvanizing). Example: Does the reaction, Al2(SO4)3 (aq) + 2 Cr (s) 2 Al (s) + Cr2(SO4)3 (aq) occur? - reactant metals are Al3+ and Cr Chromium is added to iron to make stainless steel. 5) Metathesis (double replacement) reactions - cations and anions in two ionic compounds switch places Examples: Pb(ClO3)2 (aq) + 2 KCl (aq) PbCl2 (s) + 2 KClO3 (aq) Na2SO4 (aq) + Ba(C2H3O2)2 (aq) BaSO4 (s) + 2 NaC2H3O2 (aq) There are many types of metathesis reactions. We will examine two. A.) Precipitation - When ions switch places, a precipitate (solid) forms. - We need to know solubility rules to predict if precipitate is formed. Solubility Rules Generally Soluble Compounds Anion Exceptions NO3-, C2H3O2Cl-, Br-, ISO42- None Ag+, Pb2+ Pb2+, Ca2+, Sr2+, Ba2+ Generally Insoluble Compounds Anion Exceptions S2CO32-, PO43OH-, O2- All alkali metals, NH4+, and Ca2+, Sr2+, Ba2+ All alkali metals, NH4+ All alkali metals and Ca2+, Sr2+, Ba2+ ** Note all alkali metal and ammonium compounds are soluble.** 7 Using solubility rules to predict reaction - Reaction happens if precipitate is formed. - Reaction does not happen if precipitate is not formed. Example: Does the following reaction occur and what are its products? Na2S (aq) + Fe(NO3)2 (aq) To answer question, switch ions to form potential products. Na2S (aq) + Fe(NO3)2 (aq) NaNO3 ( ) + FeS ( ) Is NaNO3 or FeS insoluble? According solubility rules, FeS is insoluble. Therefore reaction occurs Balanced molecular equation is Na2S (aq) + Fe(NO3)2 (aq) 2 NaNO3 (aq) + FeS (s) Ionic equation is 2 Na+ (aq) + S2- (aq) + Fe2+ (aq) + 2 NO3- (aq) 2 Na+ (aq) + 2 NO3- (aq) + FeS (s) Net ionic equation is Fe2+ (aq) + S2- (aq) FeS (s) Example: Does the following reaction occur and if so, what are its products? Cesium chloride (aq) + Calcium nitrate (aq) CsCl (aq) + Ca(NO3)2 (aq) CsNO3 ( ) + CaCl2 ( ) Is CsNO3 or CaCl2 insoluble? According solubility rules, both are soluble. Therefore no reaction occurs. Balanced molecular equation is 2 CsCl (aq) + Ca(NO3)2 (aq) 2 CsNO3 (aq) + CaCl2 (aq) Ionic equation is 2 Cs (aq) + 2 Cl- (aq) + Ca2+ (aq) + 2 NO3- (aq) 2 Cs+ (aq) + 2 NO3- (aq) + Ca2+ (aq) + 2 Cl- (aq) + Net ionic equation is Zip, zero, nothing, nada!!! - All the ions are spectator ions, no reaction occurs. 8 Example: Does the following reaction occur and what are its products? Ammonium sulfate (aq) + Barium acetate (aq) (NH4)2SO4 (aq) + Ba(C2H3O2)2 (aq) NH4C2H3O2 ( ) + BaSO4 ( ) Is NH4C2H3O2 or BaSO4 insoluble? Balanced molecular equation is Ionic equation is Net ionic equation is Barium sulfate is ingested by X-ray patients to improve the visibility of GI tract to X-ray radiation. B.) Neutralization - When ions switch places and water is formed. - General neutralization reaction is acid + base salt + water - Salt is general name for an ionic compound. - Acids have H+ ion. This is a very simplified view of - Bases have OH- ion. acids and bases. Example: HCl (aq) + NaOH (aq) H2O (l) + NaCl (aq) acid base water salt Ionic equation H+ (aq) + Cl- (aq) + Na+ + OH- (aq) H2O (l) + Na+ (aq) + Cl- (aq) Net ionic equation H+ (aq) + OH- (aq) H2O (l) - all neutralization reactions have this net ionic equation Example: H2SO4 (aq) + 2 LiOH (aq) 2 H2O (l) + Li2SO4 (aq) acid base water salt