CBC Controls
advertisement

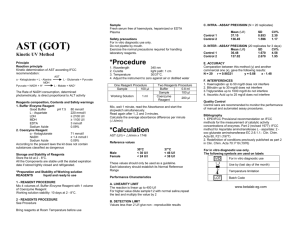
SMILE Johns Hopkins University Baltimore, MD USA Parallel Testing and Reagent Lot Validation - Guidelines Guideline Number Pro40-06 Effective Date 03/21/08 Page 1 of 1 Parallel Testing and Reagent Lot Validation - Guidelines Supersedes 1.0 Review by Review date 7-Feb-12 Parallel Testing and Reagent lot Validation Subject Heidi Hanes SMILE Comments: This document is provided as an example only. It must be revised to accurately reflect your lab’s specific processes and/or specific protocol requirements. Users are directed to countercheck facts when considering their use in other applications. If you have any questions contact SMILE. Background Information: Audit Shell: Questions pertaining to parallel testing and reagent lot validation can be found in section– Test and Control Articles item CAP Accreditation Checklist Questions pertaining to parallel testing and regent lot validation can be found in the Chemistry and Toxicology checklist – Reagents -CHM.12900, p. 25. Hematology checklist – Reagents – HEM.24575, p. 44 Background Information Clinical laboratory reagents and control materials are exposed to many variables due to conditions during transportation and storage environments in different laboratory settings. The validation of new reagents kits with old reagent kits is performed to ensure that, in spite of varying environmental conditions, there are no clinically significant differences in the results obtained when different lot numbers of reagents are used. Control materials are parallel tested to ensure that the mean of the values obtained are within the ranges specified by each manufacturer. The data gained during parallel testing should then be utilized to establish QC ranges for each individual laboratory. The procedure outlines the parallel testing and reagent lot validation testing required for different sections of the laboratory including chemistry, hematology, coagulation, flow cytometry and HIV viral load testing. Resources 1. College of American Pathologists (CAP) 2006. Commission on Laboratory Accreditation, Laboratory Accreditation Program; Laboratory General Checklist Revised 7/27/2007. Pro40-06 Parallel Testing Version#: 2.0 Page 1 of 11 SMILE Johns Hopkins University Baltimore, MD USA Parallel Testing and Reagent Lot Validation Author(s), Name & Title Document Number Jo Shim MBA, MT(ASCP) International QA/QC Coordinator (SMILE) Program Pro40-06 Effective Date 4/2/2008 SMILE Comments: This document is provided as an example only. It must be revised to accurately reflect your lab’s specific processes and/or specific protocol requirements. Users are directed to countercheck facts when considering their use in other applications. If you have any questions contact SMILE. Name, Title Signature Date Name, Title Signature Date Approved By SOP Annual Review Revision History Version # [0.0] Revision Date [dd/mm/yy] Description (notes) 2.0 17/02/10 Updated CD4/CD8 Criteria & added appendix K Name (or location) # of copies Name (or location) # of copies Distributed Copies to Pro40-06 Parallel Testing Version#: 2.0 Page 2 of 11 SMILE Johns Hopkins University Baltimore, MD USA I acknowledge that I have read, understand and agree to follow this SOP. Electronic Signature Pro40-06 Parallel Testing Version # Version#: 2.0 Date Page 3 of 11 SMILE Johns Hopkins University Baltimore, MD USA Purpose: The purpose of this procedure is to provide a procedural template for, SMILE monitored, international sites to use when developing a program for parallel testing. The procedure is intended to be used by sites as a guide while developing their own parallel testing procedures. Principle: Clinical laboratory reagents and control materials are exposed to many variables due to conditions during transportation and storage environments in different laboratory settings. The validation of new reagents kits with old reagent kits is performed to ensure that, in spite of varying environmental conditions, there are no clinically significant differences in the results obtained when different lot numbers of reagents are used. Control materials are parallel tested to ensure that the mean of the values obtained are within the ranges specified by each manufacturer. The data gained during parallel testing should then be utilized to establish QC ranges for each individual laboratory. Responsible Personnel: Responsible personnel may vary according to location but should include the following positions or their equivalents – Laboratory Manager or Director QC/QC Coordinator Department/Section Heads or Chief Technologist Staff Technologist/Technicians Precautions: Standard precautions should be followed when conducting parallel testing and reagent lot validation (refer to appropriate safety SOP). In addition, personnel performing the testing should use Personal Protective Equipment (PPE) that is appropriate for the task and follow all safety rules established for their institution. Procedure: The requirements for parallel testing of controls can vary according to the test being performed. Follow the guidelines for different testing systems as outlined below. CBC Controls: 1. The new lot of controls should ideally be run in parallel with the old lot of controls 2-3 times for 5-8 days before the old lot # expires 2. The mean for the new control and standard deviation for the new lot of the controls will be approved by the Laboratory Supervisor or QC/QA coordinator before the new control is used. Labs may have other ways of establishing QC ranges see Appendix A: Establishing Hematology QC Ranges. 3. The laboratory Director or QC/QA coordinator should review and sign off on the QC parallel testing data before the new control is put into operation. Pro40-06 Parallel Testing Version#: 2.0 Page 4 of 11 SMILE Johns Hopkins University Baltimore, MD USA Chemistry Controls: 1. The new control lot number should be run in parallel with the old lot number before it expires. The new control should be run a minimum of 20 times over 3-5 days or over a longer time period if possible. 2. The mean for the new control and standard deviation for the new lot of the controls should be approved by the Laboratory Supervisor or QC/QA coordinator before the new control is put into use. Labs may have other ways for establishing QC ranges see Appendix B: Establishing Chemistry QC Ranges. 3. The laboratory Director or QC/QA coordinator should review and sign off on the QC parallel testing data before the new control is put into operation. Coagulation Controls: 1. The procedure for the parallel testing of coagulation controls is very similar to the procedure for chemistry. The new control lot number should be run in parallel with the old lot number before it expires. The new control should be run a minimum of 20 times over 3-5 days but a longer period of time is recommended. . 2. The mean for the new control and standard deviation for the new lot of the controls should be approved by the Laboratory Supervisor or QC/QA coordinator before the new control is put into use. Reagent lot validation: All new lots of reagents should be validated by running them in parallel with the old lot numbers as indicated and the results obtained should be within the acceptability range defined. HIV EIA and other EIA Assays: 1. A minimum of 3 patient samples (negative, low positive and high positive if available) or an entire strip from a previous run are tested in parallel with QC on both the old and the new lot numbers. 2. The QC and patient results should be reproducible between the two lots (reproducibility includes both the OD readings and the interpretations). 3. The Laboratory Director, QA/QC Coordinator or designated technologist is responsible for defining acceptability limits for parallel testing. (Example: OD variance of 1SD or less and agreement on the interpretation). 4. Develop a form for the documentation of parallel testing that includes appropriate space for entering the following data: Lot numbers (old and new lot numbers) and expirations dates Results obtained from the old and new lots. Pro40-06 Parallel Testing Version#: 2.0 Page 5 of 11 SMILE Johns Hopkins University Baltimore, MD USA Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs (include QC lot numbers). Space for the person completing the parallel testing to sign and date the form and a place for a reviewer to sign and enter the date. HIV RNA PCR Quantitative Assay: 1. A minimum of 3 patient samples (not detected, low positive and high positive, if available) or an entire strip from a previous run are tested in parallel with the QC on both the old and the new kit or reagent lot number. 2. The QC and patient results should be reproducible between the two lots. 3. The Laboratory Director, QA/QC Coordinator or designated technologist is responsible for defining acceptability limits for parallel testing (typical criteria for the acceptability of Quantitative PCR assays would be that any variation should not be greater than threefold or they should be within 2 fold or 0.3 Log - HIV Prevention Trial Network (HPTN) criteria) 4. Develop a form for the documentation of reagent lot validation that includes appropriate space for entering the following data: Lot numbers (old and new lot numbers) and expirations dates Results obtained form the old and new lots. Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs (include QC lot numbers). Space for the person completing the parallel testing to sign and date the form and place for a reviewer to sign and enter the date. GC, Chlamydia, HIV PCR Qualitative Assays: 1. A minimum of 3 patient samples should be run in parallel on both the old and the new lots. 2. The QC and patient results should be reproducible between the two lots. 3. The Laboratory Director, QA/QC Coordinator or designated technologist reviews the results and confirms that there is agreement between the results for the two kits (i.e. negative results are negative on the new kit and positive results are positive). 4. Develop a form for the documentation of reagent lot validation that includes appropriate space for entering the following data: Lot numbers (old and new lot numbers) and expirations dates Results obtained from the old and new lots. Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs (include QC lot numbers). Pro40-06 Parallel Testing Version#: 2.0 Page 6 of 11 SMILE Johns Hopkins University Baltimore, MD USA Space for the person completing the parallel testing to sign and date the form and a place for a reviewer to sign and enter the date. CD4/CD8 Assay: 1. A minimum of 2 patient samples (The IQA recommends using one normal and one abnormal sample) should be run in parallel when antibody lot numbers, reagent lot numbers or reagent kits, such as truecount , lot numbers are changed. 2. The QC and patient results should be reproducible between the two lots. 3. The Laboratory Director, QA/QC Coordinator or designated technologist defines the acceptability limits for parallel testing (The IQA recommends a difference of <10% for both percent and absolute counts). 4. Develop a form for the documentation of the reagent lot validation that includes appropriate space for entering the following data: Lot numbers (old and new lot numbers) and expirations dates. Results obtained from the old and new lots. Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs Space for the person completing the parallel testing to sign and date the form and a place for a reviewer to sign and enter the date Chemistry Assays: 1. New lot numbers of chemistry reagents are run in parallel with the old lot to check the performance of the new reagent. 2. A minimum of 3 patient samples should be run on the old and new lot number. 3. The QC and patient results should be reproducible between the two lots. 4. The Laboratory Director and QA/QC Coordinator or designated technologists are responsible for defining the acceptability limits for reagent parallel testing. (Suggested acceptability limits are within +/- 1SD or within+/-10%). 5. Develop a form for the documentation of chemistry reagent lot validation that includes the following data: Lot numbers (old and new lot numbers) and expirations dates. Results obtained from the old and new lots. Pro40-06 Parallel Testing Version#: 2.0 Page 7 of 11 SMILE Johns Hopkins University Baltimore, MD USA Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs (include QC lot numbers). Space for the person completing the parallel testing to sign and date the form and a place for a reviewer to sign and enter the date CBC Analyzer Reagents 1. Comparison of materials of known value prior to and following changing or priming of new lots or shipments is required. 2. Comparison studies must be performed before or at the same time that new reagent lots are placed in service 3. Material of known value may include a patient samples or control material. 4. Background checks must be performed on inert materials such as diluent to ensure that new lots do not interfere with patient results. 5. Develop forms for the documentation of reagent lot validation and diluent background checks that include: Lot numbers (old and new lot numbers) and expirations dates. Results obtained from the old and new lots. Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. Space for the person completing the comparison testing to sign and date the form and a place for a reviewer to sign and enter the date A place for background counts to be recorded. Coagulation Reagents: PT Reagent 1. Parallel testing of a new lot of PT reagent should be completed well in advance of the expiration date of the old lot. Parallel testing of new lots of PT reagents also includes verifying the reference range, geometric mean and programming the correct ISI (international Sensitivity Index) into the coagulation analyzer. 2. To verify the reference range and geometric mean it is necessary to collect specimens from 20 “normal” patients and to run a PT with the new lot of thromboplastin reagent. 90% of the samples must fall within the current range in order to verify the range and geometric mean. If they do not, a new reference range study must be conducted to determine them. Microsoft Excel or other appropriate clinical reference range software must be used to calculate the new range and geometric mean. 3. Perform comparison studies between the old and new lot number to verify the consistency of patient results and controls. The R value for the correlation study should be >0.97. 4. Validate the PT reference range with 20 specimens. If the reference range does not validate perform a new reference range study using at least 60 specimens. Pro40-06 Parallel Testing Version#: 2.0 Page 8 of 11 SMILE Johns Hopkins University Baltimore, MD USA 5. Finally, perform a manual check of the INR and compare with the instrument generated INR result. PTT Reagents: 1. Parallel testing of PTT reagents should be conducted well in advance of the expiration of the old reagent. 2. Perform comparison studies between the old and new lot number using patient samples and controls. The R value for the correlation study should be R=>0.97. 3. To verify the PTT reference range it is necessary to collect specimens from 20 “normal” patients and to run a PTT with the new lot of reagent. 90% of the samples must fall within the current range in order to verify the range. If they do not, a new reference range study must be conducted to determine them. Microsoft Excel or other appropriate clinical reference range software must be used to calculate the new range. 4. Please note that if you monitor patients on heparin therapy you should perform a new heparin curve with each change of reagent lot. 5. Develop a form for the documentation of PT/PTT reagent lot validation that includes the following data: Lot numbers (old and new lot numbers) and expirations dates. Results obtained from the old and new lots. Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs Space for the person completing the reagent validation testing to sign and date the form and a place for a reviewer to sign and enter the date Semi quantitative Tests UA strips: 1. A minimum of 3 patient samples are run in parallel on both the old and the new lots (The three samples should demonstrate varying results across the range for different strip analytes). 2. The QC and patient results should be reproducible between the two lots. 3. Acceptance ranges should be established by the Laboratory Director, QC/QA coordinator or designated technologist. (Generally negative results should remain negative, ,positive results should give the same results or be one level up or down from the original result) 4. Develop a form for the documentation of the reagent lot validation that includes appropriate space for entering the following data: Lot numbers (old and new lot numbers) and expirations dates. Results obtained from the old and new lots. Pro40-06 Parallel Testing Version#: 2.0 Page 9 of 11 SMILE Johns Hopkins University Baltimore, MD USA Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs Space for the person completing the parallel testing to sign and date the form and a place for a reviewer to sign and enter the date Qualitative Testing (Rapid HIV test kits, urine/serum qualitative hCG etc.) 1. A minimum of 3 patient samples are run in parallel on both the old and the new lots. 2. The QC and patient results should be reproducible between the two lots. 3. The Laboratory Director, QA/QC Coordinator or designated technologist reviews the results and confirms that there is agreement between the results for the two lots (i.e. negative results are negative on the new lot and positive results are positive). 4. Develop a form for the documentation of reagent lot validation that includes appropriate space for entering the following data: Lot numbers (old and new lot numbers) and expirations dates Results obtained form the old and new lots. Criteria for acceptance and space to indicate if the results obtained on the new lots were acceptable. QC values on both runs Space for the person completing the parallel testing to sign and date the form and a place for a reviewer to sign and enter the date References Westat Checklist General required elements – Parallel Testing Westgard website - www. Westgard.com Mercy Medical Center –Baltimore, Maryland: Yearly Coagulation Lot changes Grove N., Rotzoll, K. CLIA Corner: Prothrombin Time and INR Testing. University of Iowa Hygienic Laboratory Appendices Appendix A: Establishing Hematology QC Ranges Appendix B: Establishing Chemistry QC Ranges Examples of Forms for Parallel testing Documentation Appendix C: PCR Reagent Lot Validation Form Appendix D: Qualitative Reagent Lot Validation Form Appendix E: EIA Test Reagent Lot Validation Form Pro40-06 Parallel Testing Version#: 2.0 Page 10 of 11 SMILE Johns Hopkins University Baltimore, MD USA Appendix F: Urine hCG Reagent Lot Validation Form Appendix G: PT Reagent Lot Validation Form Appendix H: PTT Reagent Lot Validation Form Appendix I: Hematology Reagent Lot Validation Form Appendix J: Chemistry Reagent Lot Validation Form Appendix K: Flow Cytometry Reagent Lot Validation Form Pro40-06 Parallel Testing Version#: 2.0 Page 11 of 11