How to clone an ion channel (notes on slides 26
advertisement

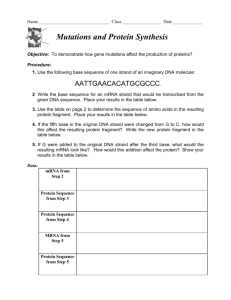
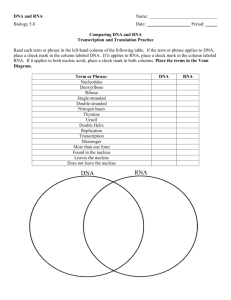
How to clone an ion channel: to be read in conjunction with the PowerPoint slides on “Finding the sequence of an ion channel” Slide 26 We used to (1970s till mid 1980s) sequence proteins by directly identifying the amino acids starting at the N terminus. But this only worked for very short sequences (up to about 10 amino acids). It became easier to get the sequence of DNA or (indirectly) messenger RNA. This makes sequencing much easier, as shown inthe other slides. The illustration shows the basic mechanisms of protein synthesis, which will be familiar to you. Chromosomal DNA sequence determines the sequence of messenger RNA, which determines the protein sequence. Slide 27 A reminder of the “genetic code”. The messenger RNA (mRNA) strand is “complementary” to the DNA. Each group of three nucleotides (called a codon) in the messenger RNA codes for one amino acid. The first one here (reading from the 5’ end of the mRNA, placed at the left) is methionine, coded by AUG: this is the first amino acid in all proteins (unless it is later removed), so that AUG can be considered as the “start codon”. In the process of translation, a ribosome “searches for” the first AUG codon (usually the first, although there are a few exceptions) then attaches amino acids in sequence, one amino acids for each group of 3 nucleotides. Slide 28 To work out the mRNA sequence, we make use of the fact that all mRNA molecules get a “tail” of multiple adenosines (AAAAAAA) added to them. So if we use a short stretch of TTTTTTT attached to a bead, we can purify mRNA. This step is not always necessary because the next step also uses the TTTTTTT. Slide 29 Now we use the mRNA sequence as a “template” to make a strand of DNA complementary to the mRNA. We use a TTTTTTTT “primer” (a primer is a short DNA [rarely RNA] molecule that is used to start the process of nucleic acid synthesis) and the enzyme “reverse transcriptase” (actually it’s RNA-dependent DNA polymerase, but the name “reverse transcriptase” was coined for it and it stuck). Reverse transcriptase uses the RNA (brown) to determine the sequence of a new strand of DNA (blue). Because this DNA strand is complementary to the mRNA, it is called complementary DNA or cDNA. The RNA-DNA hybrid in the middle of this slide is used as the basis for a doublestranded DNA molecule: the first strand of the DNA is used as the template to make the “second strand”, which is complementary to it. Slide 30 To make a lot of this sequence, it is incorporated into a plasmid, which is put into bacteria (a plasmid is a little circular piece of DNA which the bacteria can copy and pass on to their descendants). “Colonies” of bacteria grow from each single bacterium, and the whole colony expresses the same plasmid. Because the bacteria are identical, the colony is referred to as a “clone” and the process just described is known as “cloning”. Slide 31 The collection of colonies is “screened” to find which colony or colonies is/are expressing the sequence of interest. That colony can be grown on a large scale and the plasmid isolated. Slide 32 The plasmid, once isolated, can be used to make mRNA by a process called “in vitro transcription” - transcription because we’re using a DNA sequence to make RNA; in vitro (Latin - “in glass”) because it happens in a plastic tube rather than in a cell. The mRNA can be injected into oocytes (egg cells) from the toad Xenopus laevis. These are very large cells (~1 mm diameter) and easy to record. Alternatively (not shown), and more usually nowadays, we use mammalian cultured cells instead of Xenopus oocytes, and just inject the plasmid, leaving the cell to make the mRNA. Injecting the mRNA into oocytes, or transferring the plasmid into mammalian cells, will cause theoocyte or cell to “express” (i.e. synthesise) the ion channel or other protein that’s coded for by the mRNA sequence we started with. Slide 33 The “screening” in slide 6 assumes that we actually know what sequence we are looking for. This is not an easy step when it is being done for the fisr time with a novel protein. The example we will look at is the Drosophila potassium channel called Shaker. Slide 34 The Shaker mutation of Drosophila causes flies to shake their legs when anaesthetised with ether. Physiological experiments on these flies indicated that the Shaker mutation affected an ion channel. Genetic method in Drosophila allow us to identify what region of a chromosome is affected by a given mutation, and to isolate short sequences of DNA known to be from these regions. So it was known that the Shaker mutation affected a region of a chromosome (the X chromosome) near to a known DNA sequence called 16F. Slide 35 Starting with a DNA clone from 16F, a “library” (a collection of clones of chromosomal DNA) was probed to find matching sequences. Once a matching sequence was found (which would be a stretch of DNA a little further along than 16F), part of this new sequence was used a a probe, which moved the known region a little further along, and so on. This is known as a “chromosome walk” because we are “walking” along a chromosome. Slide 36 A graphical representation of the “chromosome walk” technique. The result is the sequence of a long stretch of chromosomal DNA. This is not in itself the DNA sequence that codes for the ion channel, because it contains both “exons” (sequences that code for the ion channel) and “introns” (non-coding sequences, that are cut out of the mRNA before protein synthesis). This sequence is used as the basis for making “probes (short sequences of DNA) that are used to search a second library. This second library, instead of being prepared from chromosomal DNA, is prepared from messenger RNA by the method shown in slides 3-6. The DNA sequences present in this second library thus correspond exactly to mRNA sequences that directly code for all the proteins that the fly is expressing. When we isolate a clone from that library, it is thus a clone containing coding sequence for the Shaker potassium channel. Several clones would be isolated, and among the longest we would hope to find a “full length” clone. Slide 37 Once the “full length” clone is isolated, we get the full cDNA sequence (the “second strand” sequence from slide 4 is usually the one that is shown - it is equivalent to the mRNA sequence, except that uracil in RNA appears as thymidine in DNA, so all the T’s here would be U’s in the mRNA). Slide 38 We can “translate” the sequence in slide 12, knowing the “genetic code”, to give the amino acid sequence of the ion channel, as shown in in the lecture.