Mech 473 Homework Assignment #1
advertisement

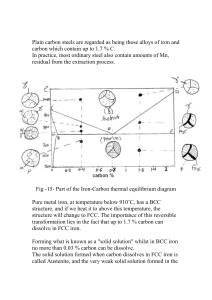
Mech 473 Homework Assignment #2 1. a) Determine the constants c and n in a sigmoidal equation that describes the rate of phase transformation in the figure below at 135 oC. b) Determine the activation energy for the phase transformation from the sigmoidal curves. Hint: the rate of transformation is the reciprocal of the time required for half of the transformation to occur. Use the Arrhenius equation. (4 marks) sigmoidal equ’n is: n = 3.1 If f = 0.6, then t = 10 min and 3.1 1 0.6 exp c10 c 7.28 10 4 2. A steel contains 10% cementite and 90% ferrite at room temperature. Estimate the carbon content of the steel. Is the steel hypoeutectoid or hypereutectoid? (1 mark) 0.9 6.67 x ; x 0.92%C 6.67 0.02 Since x > 0.77, the steel is hypereutectoid. 3. For a Fe – 1.15 %C alloy, determine a) the temperature at which austenite first begins to transform on cooling, b) the primary microconstituent that forms, c) the amount of each phase present at 726 oC and e) the composition and amount of each microconstituent present at 726 oC. (2.5 marks) 4. A steel that is heated until 85% austenite has a carbon content of 1.05%. Estimate the temperature and the overall carbon content of the steel. (1 mark) In order for the to contain 1.05%C, the austenitizing temperature must be about 845 oC (from the tie line). At this temperature: 6.67 x 0.85 ; x 1.893%C 6.67 1.05 5. A steel that is heated until 85% austenite has a carbon content of 0.5%. Estimate the temperature and the overall carbon content of the steel. (1 mark) In order for the to contain 0.5%C, the austenitizing temperature must be about 770 oC (from the tie line). At this temperature: x 0.01 0.85 ; x 0.43%C 0.5 0.01 6. Compare the interlamellar spacing and the yield strength when a eutectoid steel is isothermally transformed to pearlite at a) 700 oC and b) 600 oC. Use figures provided in class. (1 mark, ½ each) We can find the interlamellar spacing from the figures provided in the class notes (below) and then use this spacing to find the strength from the additional figure. a) = 7.5 x 10exp(-5) cm 1/ = 13,333; YS = 200 MPa (29,400 psi) b) = 1.5 x 10exp(-5) cm 1/ = 66,667; YS = 460 MPa (67,600 psi) 7. A steel containing 0.3% C is heated to various temperatures above the eutectoid temperature, held for one hour and then quenched to room temperature. Determine the amount, composition and hardness of any martensite that forms when the heating temperature is 728 oC and 790 oC. Determine %C in at temperature to determine % Martensite and then use figures provided in class for hardness. (2 marks) 728o C : 0.77%C %M 0.3 - 0.0218 100% 37.2% HRC 65 0.77 0.0218 790o C C %M 0.3 - 0.02 100% 84.8% HRC 58 0.35 0.02 Hardness RC 8. A steel microstructure contains 92% martensite and 8% Fe3C where the composition of the martensite is 1.10%C. Determine the temperature from which the steel was quenched and the carbon content of the steel. (1 mark) In order for the (and therefore martensite) to contain 1.10%C, the austenitizing temperature T = 865 oC. Then: at T 865o C M 0.92 6.67 - x , x 1.55%C 6.67 1.10 9. Calculate the amounts of ferrite, cementite, primary microconstituent (primary and Fe3C) and pearlite in the following the two steels designated as 1015 and 1095. (4 marks) 1015 6.67 - 0.15 100% 97.8% Fe3C 2.2% 6.67 0 0.77 - 0.15 primary 100% 82.9% pearlite 17.1% 0.77 0.0218 1095 6.67 - 0.95 100% 85.8% Fe3C 14.2% 6.67 0 0.95 - 0.77 primary Fe3C 100% 3.1% pearlite 96.9% 6.77 0.77 10. Estimate the AISI-SAE number for steels having the following microstructures, a) 38% pearlite – 62% primary ferrite and b) 97% ferrite – 3% cementite. (2 marks) 38% pearlite 62% primary ferrite 0.77 - x 62% 100; x 0.306%C 1030 steel 0.77 0.0218 97% ferrite 3% cementite 6.67 - x 97% 100; x 0.200%C 1020 steel 6.67 0 11. We wish to produce a martensitic stainless steel containing 17% Cr. Recommend a carbon content and austenizing temperature that would permit us to obtain 100% martensite during the quench. What microstructure would be produced if the martensite were then tempered until the equilibrium phases formed? The Fe-Cr-C phase diagram is provided below for your assistance. (3 marks) We select a combination of a carbon content and austenitizing temperature that puts us in the all-austenite region of the Fe-Cr-C phase diagram. One such combination is 1200 oC and 0.5% C. If a 0.5%C steel is held at 1200 oC to produce all austenite, and then quenched, 100% martensite will form. If the martensite is tempered until equilibrium is reached, the two phases will be ferrite and M23C6 exists. The M23C6 is typically Cr23C6. 12. You would like to produce a gray cast iron that freezes with no primary austenite or graphite. If the carbon content in the iron is 3.5%, what percentage of silicon must you add? (1 mark) We get neither primary phase when the carbon equivalent (CE) is 4.3%. Thus, CE = 4.3 = %C + (1/3)%Si = 3.5 + (1/3)%Si or %Si = 2.4 13. What is the primary source (alloying addition) of hardening (strengthening) during heat-treating of steels? (1 mark) The primary source of strengthening of steels is the martensite transformation and the presence of carbon in the composition that distorts the martensite structure. The strength of the quenched martensite depends solely on the carbon content and not on alloy content. In fact, high alloy steels and other materials such as titanium will transform to martensite but will not produce the hardness effect observed in plain carbon steels. For high alloy steels such as tool steels, secondary strengthening may also b e obtained during tempering with precipitation of alloy carbides. 14. What are the unique characteristics of the martensite transformation? Explain the significance of each characteristic. (2 marks) The unique characteristics are a) diffusionless transformation, b) it is a displacive pr shear-like transformation and c) it involves a change in crystal structure. The diffusionless characteristic signifies two things: 1) the amount, and 2) the composition of the old or parent phase is the same as the martensite. The displacive or shear-like transformation signifies that a large volume of the old phase may transform at one time. The change in crystal structure signifies that the characteristics of the martensite are different from the old phase. An increase in volume of the martensite phase over the old phase is a very beneficial characteristic that increases the fracture toughness of a material, such as in ceramics. 15. What are high-speed tool steels and their main applications? What are hot-work tool steels and their uses? What are cold-work tool steels and their uses? What are waterhardening tool steels and their uses? (4 marks) High speed tools steels are used for high cutting speeds such as drills, mill cutters, taps and others. Hot-work tool steels are intended to withstand combinations of heat, pressure, and abrasion associated with shearing, punching or forming of metals during manufacturing at high temperatures. Cold-work tool steels are those intended for applications that do not require prolonged or repeated heating in the range above 205-260 o C. Shock resisting tool steels are those intended for applications requiring toughness and resistance to shock-loading such as hammers, chisels, punches, driver bits and others. Water hardening tool steels are shallow hardened and have relatively low resistance to softening. They are suitable for woodworking tools, hand-metal cutting tools such as taps and reamers and cutlery. 16. What are stainless steels and the five different types of stainless steels? (2 marks) Stainless steels are alloys of iron with a minimum addition of 10.5%Cr, which imparts a passivity and prevents the rusting of the steel. The five types of stainless steels are ferritic, austenitic, martensitic, duplex and precipitation hardening steels. 17. What is the most significant factor used in the selection of stainless steels, and the predominant type of stainless steel used in the market? (2 marks) Stainless steels are used predominantly for their corrosion resistance. Thus, corrosion resistance rates the highest with their mechanical properties coming second. The most predominant type is the 304 austenitic stainless steel and selection of stainless steel starts by considering first if 304 can do the job or not. Other grades are chosen when 304 needs an upgrade in other properties. 18. What is meant by stabilization and its significance in regards to stainless steels? What is another method to reduce the sensitization of stainless steels? (2 marks) Stabilization in stainless steels refers to the process of intentionally combining the carbon content in the steels with more stable carbide formers such as Ti or Nb other than chromium to avoid the depletion of chromium around grain boundaries that results in intergranular corrosion and failure. The other method to reduce sensitization of stainless steels is to lower the carbon content. This can be done by specifying grades with “L” at the end, such as 304L versus 304. 19. What is a quick and easy way to differentiate an austenitic stainless steel from a ferritic, martensitic or duplex stainless steel? (1 mark) An austenitic stainless steel will not attach a magnet, whereas the others will. An austenitic stainless steel can also be used in a microwave.