Voice Related Devices and Respiratory Equipment
advertisement

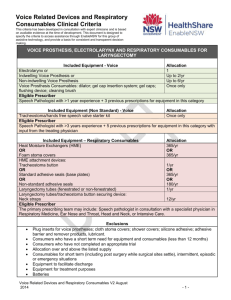
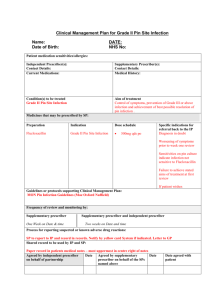
Equipment Request Form Voice Related Devices and Respiratory Consumables New Request Amendment to Existing Request 1. CLIENT INFORMATION Client Name Last Name ENABLE # First Name Title Mr Mrs Ms Miss Other Date of birth: Address Suburb Postcode Phone Mobile Contact person (if not client) Relationship Partner Parent Phone Mobile Email Relative Carer Friend Email Diagnosis Date of surgery: Date of Discharge: N/A 2. EQUIPMENT RECOMMENDATION New Product Replace ment Product Product name and Code Supplier Supply Allocation* Electrolarynx one per consumer Indwelling Voice Prosthesis 2/year OR Non Indwelling Voice Prosthesis: 6/year Trache-oesophageal Dilator: one per consumer Gel Cap Insertion Kit: one pack per consumer Gel Caps: one pack per consumer Cleaning brushes/ flushing device: one pack per consumer Tracheostoma/Hands Free Speech Valve Starter Kit: Cost one per consumer Justification required – see below ERF_ EnableNSW _ Voice Related Devices and Respiratory Consumables July 2014 Page 1 of 3 Equipment Request Form Name: Date of Birth: Heat Moisture Exchangers (including foam stoma covers): 365/year Tracheostoma Button: 1/year OR Standard Adhesive Seals: 365/year OR Non standard Adhesive Seals: 180/year Justification required – see below Laryngectomy Tube: 1/year Neck Straps: 12/year TOTAL COST (Office Use) $ *This is the standard annual allocation and actual quantity may vary depending on packaging 4. EQUIPMENT JUSTIFICATION a) Has the surgical site stabilized? Yes No b) Will the equipment be required on a permanent basis (≥ 12 months)? Yes No c) Is the consumer able to use the recommended equipment safely and appropriately, Yes No Yes No Yes No including care, maintenance and emergency planning in the event of equipment failure? d) Is the consumer/carer aware of the annual allocation through EnableNSW and has information regarding purchase of additional supplies if required? e) Has a trial on all the requested equipment been completed? f) If requesting increased annual allocation of indwelling voice prosthesis, and/or provision of non-standard adhesive seals, and/ or a tracheostoma/hands free speech valve, please provide clinical justification to support the request. Please refer to Voice Related Devices and Respiratory Consumables Clinical Criteria. N/A ERF_ EnableNSW_ Voice and Respiratory Devices Aug 2013 Page 2 of 3 Equipment Request Form Name: Date of Birth: g) Are any changes anticipated that may impact on this equipment request? (e.g. change to size/type of device) No change anticipated. Yes. If yes, please recommend appropriate supply schedule. h) i) i) Is the consumer aware of and in agreement with this equipment request? Yes Date agreement received: No N.B. Application will only be processed with consumer/carer agreement. A copy of the equipment request has been provided to the consumer. Yes Date No If no, why? Name and contact details of local speech pathologist (if different to prescriber): OR See prescriber details below 5. DELIVERY INFORMATION a) Who should be notified when the equipment is ready to be delivered? Prescriber Consumer/contact person Other Provide contact name, relationship, phone, email b) Delivery address for equipment Consumer’s home address Other, provide details c) Delivery Instructions Yes No If yes, details: 6. PRESCRIBER DECLARATION Please provide the name, address and contact details of the prescriber Name: Address: Service: Days/hours available: Qualification/role: Phone: Email: DECLARATION I declare that I have assessed the consumer in consultation with an appropriate multidisciplinary team and have the required qualification and level of experience to prescribe this equipment according to the Professional Criteria for Prescribers. Signature: Supervisor name, name of service, address, phone, email: (if required) Date: Signature of supervisor (if practical): Qualification: Days/Hours available: Date: NB: Incomplete forms will delay processing of the application. Please ensure all contact details are provided. ERF_ EnableNSW_ Voice and Respiratory Devices Aug 2013 Page 3 of 3