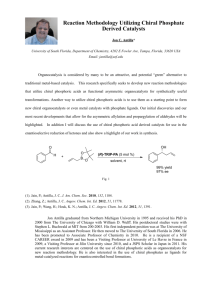
Asymmetric Counteranion Directed Enantioselective
advertisement

RESEARCH PROPOSAL Asymmetric Counteranion Directed Enantioselective Radical Cyclizations Ziqing Qian Abstract: This research proposal summarizes scientific context of enantioselective radical reactions, especially chiral Lewis acid mediated enantioselective radical cyclizations. This proposal based on Prof. Dan Yang’s research of enantioselective group-transfer tandem radical cyclization reactions, aims at employing Asymmetric Counteranion Directed Catalysis (ACDC) concept to study some unsolved problems in this area. Keywords: asymmetric counteranion directed catalysis·radical cyclization. Lewis acid catalysis Introduction molecular construction (cyclizations) have been reported.[5-9] These reactions can be classified into For the requirement of pharmaceutical and two types by the nature of coordination with a agrochemical synthesis, asymmetric synthesis has Lewis acid (Type A and B, ML* = chiral Lewis attracted much attention. Chiral compounds are acid). typically accessed from either Nature’s “chiral pool,” by resolution of a racemate or by means of an enantioselective transformation mediated by a chiral catalyst.[1] Radicals, as one major kind of intermediates, have been recognized as highly reactive short-lived species that are difficult to tame. This misconception, which hindered their use in stereoselective reactions, has long since been Enantioselective Radical Cyclizations negated, and the synthetic utility of radicals has been properly recognized. There are many good A recent work on enantioselective type A radical sources of literature discussing radical chemistry cyclizations was reported by Takemoto’s group and the appropriate methods for its application in (Scheme 1).[9] A suitable combination of a chiral organic synthesis.[2] The study of stereochemistry in radical-mediated reactions has been of great importance to organic synthesis.[3] Enantioselective Radical Reactions Researchers achieved success in different types of asymmetric radical reactions: atom/group- transfer reactions, reductive alkylations, tandem reactions, oxidations, polymerizations, etc.[4-5] Particularly chiral Lewis acids promoted enantioselective carbon-carbon bond construction from radical intermediates have attracted much interests. Stereocontrol in both intermolecular reactions (addition reactions, allylation, etc.) and intra- Ziqing Qian Department of Chemistry, East China Normal University Shanghai, China RESEARCH PROPOSAL Lewis acid and hydroxamate ester leads to highly cyclization of 6 proceeded smoothly to give enantioselective reactions of 1. Although this new cyclized product 8 in good enantioselectivity when approach of utilizing hydroxamate esters has chiral ligand 7 and Mg(ClO4)2 were employed.[7b] achieved PhSe-group-transfer radical cyclization of 9 also highly enantioselective additiona proceeded with 89% ee and better yield.[8a] In some powerful synthetic approach to γ-latams, prepare of cases, high enantioselectivity was achieved using hydroxamate esters is difficult. Some other enantio- 0.3 equivalents of the chiral Lewis acid catalyst, but selective cyclizations have been achieved by it is still not a perfect methodology, considering Nishida’s group, Yang’s group, Ishii’s group, limited substrate scope, large catalyst load, toxicity Curran’s group, Bach’s group, and Takemoto’s of PhSe-substituents, and reaction temperature cyclization-trapping group. reaction that provides [6-8, 10-11] (-78℃) in this system. From 2002 to 2006, Yang and coworkers focused on the asymmetric Lewis acid-catalyzed Enantioselective tandem or cascade radical bromo- cyclizations attract much interest, since highly and phenylseleno- transfer radical cyclizations of a functionalized compounds with multiple stereo- serious of α-substituted β-keto esters. Using centers are provided, and multiple carbon–carbon β-keto esters bidentate coordinating substrates for bonds and some tertiary chiral centers are formed in the design of enantioselective radical cyclization, a single operation; thus, a number of extensive they of investigations into sequential radical reactions were α-substituents of β-keto esters, and found that reported in recent years.[12] Yang’s group also although high enantioselectivity (up to 94% ee) reported Lewis acid-catalyzed atom-/group-transfer could be achieved both in the case of bromo- and tandem radical processes of α-radical species β-keto phenylseleno- β-keto esters in type B cyclization esters to give monocyclic or bicyclic products in a (Scheme 2), the use of bromide unfortunately high efficient, regioselective, and stereoselective [7b, 8a,b] manner.[8] For instance, the type B cyclization has systematically afforded lower yield. studied the [7-8] effect Atom-/group- transfer been applied to tandem cyclization (Scheme 3) by Yang and co-workers.[8b] Tandem reaction of 11 could be performed with ligand 7 and Mg(ClO 4)2. Good enantioselectivity was observed when reaction was carried out in toluene at -20℃, but the yield of the product 12 was low. Recently, Yang’s group also reported that the phenylseleno- group-transfer reaction was ideal in terms of both reaction efficiency and enantioselectivity for the tandem cyclization (Scheme 4).[8a] However, yield is still low, and introduction of PhSe-group, low Ziqing Qian Department of Chemistry, East China Normal University Shanghai, China RESEARCH PROPOSAL My Strategy As introduced above, ACDC strategy has emerged as a promising strategy in Lewis acids-mediated asymmetric catalysis. However, it has not been employed in catalytic asymmetric radical reactions. In this light, I wish to introduce ACDC strategy into enantioselective radical reactions (Scheme 5). temperature (-78℃), high catalyst load, and add of activated molecular sieves are necessary to obtain the enantioselectivity. Chiral Counteranion Strategy In general, chiral catalysts rely on covalent (dative or nondative) bonds between the reactive site and the chiral moiety. An alternative approach is the induction of asymmetry by interaction of the cationic catalyst with a chiral counteranion. Highly efficient chiral catalytic transformations involving chiral anionic catalysts have been reported in phase-transfer catalysis, Brønsted acid catalysis, organocatalysis, catalysis As can be expected, chiral counteranions could tune Intramolecular hydroalkoxylation of the chiral space around Lewis acids, either used allenes proceeded with a high level of enantio- alone or in combination with chiral ligand, just like selectivity to give pyrrolidine products with a its applications in transition-metal asymmetric (Scheme 5). [13] and transition-metal synthesis. It is interesting to examine if the introduction of ACDC concept, using chiral counteranion alone or in combination with chiral Lewis acid, will decrease the catalyst loading, achieve high enantioselectivity and high yield under mild conditions, or expand the substrate scope, for example, using monodentate substrates instead of bidentate substrates. . My Aims in PhD Research a cationic gold complex and the chiral phosphate counteranion example provides To develop novel transition metal promoted asymmetric radical reactions directed towards promising method towards introducing asymmetric polycyclic units based on chiral counteranion counteranion strategy into Lewis acid catalyzed strategy. Ziqing Qian This A. a reactions. 15. B. To study the applications of the methodology Department of Chemistry, East China Normal University Shanghai, China RESEARCH PROPOSAL Conclusion in natural products and bio-active molecules synthesis. C. To study reaction mechanisms, which benefit the development of .new reactions or catalysts. To sum up, since my initial interests towards enantioselective radical reactions and chiral counteranion strategy, I would very like to try my My Academic Background proposal in Prof. Yang Dan’s laboratory for my PhD research period. As a PhD applicant this year, I I have achieved an outstanding academic would like to devote myself to challenging and performance of 3.75 major GPA, and ranked top pioneer work – do best research in my PhD life. In 3% in our department. Combining with extensive this light, working in your eminent program is no research experiences in organic chemistry, I have doubt the best way to turn my dream into reality. prepared myself to challenging and pioneer work in my PhD life. My present research focus on asymmetric References and Notes transition metal catalysis: asymmetric nucleophile- 1. J. Mulzer, in Comprehensive Asymmetric Catalysis, E. N. assisted cyclization of enynones. I synthesized Jacobsen, A. Pfaltz, H. Yamamoto, Eds. (Springer, Berlin, cationic Au (Ⅰ) and Au (Ⅲ) complexes with chiral 1999), vol. I, chap. 3. binaphthol-derived phosphate chiral anions, tested 2. For selected examples of radical reactions, see: a) Renaud, P., their performance, and achieved moderate enantio- Sibi, M. P., Eds. Radicals in Organic Synthesis; Wiley-VCH: selectivity. From the practice, I noticed the New York, 2001; Vols. 1 and 2. b) Parsons, A. F. An importance of chiral counteranion strategy in Introduction to Free Radical Chemistry; Blackwell Science: asymmetric synthesis because of the importance of Oxford, 2000. c) Alfassi, Z. B. General Aspects of the anions in organic reactions and high efficiency of Chemistry of Radicals; Wiley: New York, 1999. d) Fossey, J.; chiral counteranion's chiral induction. I also Lefort, D.; Sorba, J. Free Radical in Organic Chemistry; believed the combination of transition metal Wiley: New York, 1995. e) Curran, D. P. In Comprehensive catalysis and organocatalysis is the future trend of Organic Synthesis; Trost, B. M., Fleming, I., Semmelheck, M. asymmetric synthesis. This practice equipped me F., Eds.; Pergamon: Oxford, 1991; Vol. 4, p 715. f) Giese, B. with a Radicals in Organic Synthesis. Formation of Carbon-Carbon deeper understanding of asymmetric synthesis from perspectives of stereochemistry, which is critical in my future research of chiral counteranion directed asymmetric radical reactions. Bonds; Pergamon: Oxford, 1986. 3. For selected examples of stereochemistry of radical reactions, see: a) Radicals in Organic Synthesis, Vols. 1 and 2 (Eds.: P. I also performed some other organic chemistry Renaud, M. P. Sibi), Wiley-VCH, Weinheim, 2001; b) B. research projects: “Synthesis of truxene-derived Giese, B. Kopping, T. Gçbel, J. Dickhaut, G. Thoma, K. J. derivatives for nanosized π-conjugated molecules”, Kulicke, F. Trach, Org. React. 1996, 48, 301; c) D. P. Curran, “Development of novel reactions of enynones with N. A. Porter, B. Giese, in Stereochemistry of Radical different nucleophiles” (Please refer to my CV). Reactions: Concepts, Guidelines, and Synthetic Applications, From these experiences, I got to know how to identify reaction conditions from analysis of reaction mechanisms, and reactions, see: a) Sibi, M. P.; Rheault, T. R. In Radicals in electronic effects. I also obtained evaluated Organic Synthesis; Renaud, P., Sibi, M. P., Eds.; Wiley-VCH: hands-on synthesis: Weinheim, 2001; Vol. 1, pp 461. b) Porter, N. A.; Giese, B.; experiences especially in organic functional group transformations. steric VCH, Weinheim, 1996. 4. For general information details for enantioselective radical Curran, D. P. Acc. Chem. Res. 1991, 24, 296. 5. For selected examples of intramolecular carbon-carbon Ziqing Qian Department of Chemistry, East China Normal University Shanghai, China RESEARCH PROPOSAL construction radical reactions, see: a) Sibi, M. P., Manyem, S., Synthesis, I. Ojima, Ed. (Wiley-VCH, New York, ed. 2, 2000), Zimmerman, Chem. Rev. 2003, 103. 3263. b) G. Bar, A. F. chap. 10, pp. 727–755. b) G. Lelais, D. W. C. MacMillan, Parsons, Chem. Soc. Rev. 2003, 32, 251; c) Sibi, M. P.; Porter, Aldrichim. Acta. 2006, 39, 79. c) M. S. Taylor, E. N. Jacobsen, N. A. Acc. Chem. Res. 1999, 32, 163. d) M. P. Sibi, N. A. Angew. Chem. Int. Ed. 2006, 45, 1520. d) S. Mayer, B. List, Porter, Acc. Chem. Res. 1999, 32 163; e) P. Renaud, M. Angew. Chem. Int. Ed. 2006, 45, 4193. e) G. L. Hamilton, E. J. Gerster, Angew. Chem. 1998, 110, 2704; Angew. Chem. Int. Kang, M. Mba, F. D. Toste, Science 2007. 317, 496. f) S. Ed. 1998, 37, 2562; Mukherjee, B. List, J. Am. Chem. Soc. 2007, 129, 11336. g) 6. M. Nishida, H. Hayashi, A. Nishida, N. Kawahara, Chem. Commun. 1996, 579. M. Rueping, A. P. Antonchick, C. Brickmann, Angew. Chem. Int. Ed. 2007, 46, 6903. h) N. J. A. Martin, B. List, J. Am. 7. For a selected examples of enantioselective radical reactions Chem. Soc. 2006, 128, 13368. i) T. Akiyama, J. Itoh, K. reported by Yang et al., see: (a) D. Yang, Q. Gao, B.-F. Zheng, Fuchibe, Adv. Synth. Catal. 2006, 348, 999. j) D. B. and N.-Y. Zhu, J. Org. Chem. 2004, 69, 8821. b)Yang, D.; Gu, Llewellyn, D. Adamson, B. A. Arndtsen, Org. Lett. 2000, 2, S.; Yan, Y. L.; Zhu, N. Y.; Cheung, K. K. J. Am. Chem. Soc. 4165. 2001, 123, 8612; 8. For selected examples of enantioselective tandem radical reactions reported by Yang et al., see: a) D. Yang, B.-F. Zheng, Q. Gao, S. Gu, N.-Y. Zhu, Angew. Chem. 2006, 118, 261; Angew. Chem. Int. Ed.2006, 45, 255.b) Yang, D.; Gu, S.; Yan, Y. L.; Zhao, H. W.; Zhu, N. Y. Angew. Chem. 2002, 114, 3143; Angew. Chem., Int. Ed. 2002, 41, 3014. c) D. Yang, Q. Gao, O-Y. Org. Lett. 2002, 4, 1239-1241. 9. K. Hiroi, M. Ishii, Tetrahedron Lett. 2000, 41, 7071. 10. a) H. Miyabe, R. Asada, A. Toyoda, Y. Takemoto, Angew. Chem. 2006, 118, 5995; Angew. Chem. Int. Ed. 2006, 45, 5863. b) H. Miyabe, Y. Takemoto, Chem. Eur. J. 2007, 13, 7280. 11. Transfer of chirality in radical cyclization was reported. See: D. P. Curran, W. Liu, C. H.-T. Chen, J. Am. Chem. Soc. 1999, 121, 11012. 12. a) T. Aechtner, M. Dressel, T. Bach, Angew. Chem. 2004, 116, 5974; Angew. Chem. Int. Ed. 2004, 43, 5849; b) M. Dressel, T. Aechtner, T. Bach, Synthesis 2006, 2206. 13. For selected recent examples of tandem or cascade radical reactions, see: a) K. Miura, M. Tojino, N. Fujisawa, A. Hosomi, I. Ryu, Angew. Chem. 2004, 116, 2477; Angew. Chem. Int. Ed. 2004, 43, 2423; b) M. Tojino, Y. Uenoyama, T. Fukuyama, I. Ryu, Chem. Commun. 2004, 2482; c) Y. Uenoyama, T. Fukuyama, O. Nobuta, H. Matsubara, I. Ryu, Angew. Chem. 2005, 117, 1099; Angew. Chem. Int. Ed. 2005, 44, 1075. 14. For selected examples of chiral counteranion strategy in phase-transfer catalysis, organocatalysis, and transition-metal catalysis, see: a) M. J. O’Donnell, in Catalytic Asymmetric Ziqing Qian Department of Chemistry, East China Normal University Shanghai, China