Identification of Biological Molecules
advertisement

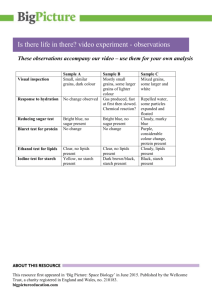
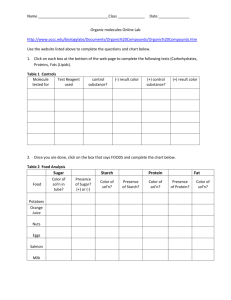
Identification of Biological Molecules All living things are essentially composed of the same basic groups of molecules. The four types of carbon-based molecules you will perform chemical tests for in this laboratory exercise are carbohydrates (sugars and starch), lipids, amino acids, and proteins. These molecules come from what you eat, so many of the samples you test will be food products. Objectives When you have finished this lab, you will be able to 1. list the four groups of biological molecules. 2. explain the necessity of using controls in experiments. 3. define all boldface terms. 4. distinguish positive from negative test results. Note: Goggles must be worn for all parts of this exercise. Procedures: Molecules that are built the same way can be expected to behave nearly the same in a chemical reaction. In this lab you will perform tests to detect the presence of several classes of molecules and macromolecules (large molecules). When performing chemical tests, first a set of standards or controls, is done against which you may compare your results. A negative control uses a substance that will not react with the chemicals. This will show you what a negative result looks like. A positive control is a substance that will react with the chemicals. It will show you a positive result. Be sure you follow directions and carefully measure out the proper amount of each material. Carbohydrates: Carbohydrates like sugars and starches are important sources of energy in your diet. Foods like pasta, bread and potatoes are examples of the complex carbohydrates dieticians suggest we eat more of while lessening our intake of refined “quick-energy” sugars like sucrose (table sugar) and high fructose corn syrup, a component of many sweetened foods and drinks. Spring 2006 1 Sugar Test Simple sugars can be detected using Benedict’s test. Benedict’s reagent changes color (after heating) in the presence of these molecules. However, some other sugars and starches are structurally different enough that they do not give a positive test (a color change). Perform the following tests: 1. Number 7 test tubes and add your initials. 2. Add equal amounts of Benedict’s reagent and the proper sample (see Table 1). 3. Heat in the boiling water bath for 5 to 10 minutes. Remove the hot tubes with test tube holders and place them in your test tube rack. 4. Record your results and interpretations (what those results mean; e.g., does the sample contain sugar?) in Table 1. The color you get also indicates how much simple sugar is present. For instance, blue-green = small amount, yellow = medium, and red-orange = a large amount. Tube # 1 2 3 4 5 6 7 Table 1. Results of Benedict’s test for sugars Sample Material Final Color Interpretation of Results Water Clear blue No simple sugars present Glucose Onion juice* Potato juice* Sucrose Mashed green banana* Mashed ripe banana* *To make a sample of these items, cut a small cube of the material, add a small amount of water and mash it with a mortar and pestle. Answer the following questions about this test: What is the negative control in this test? _____________________________________________ What is the positive control in this test?______________________________________________ Which banana (ripe or green) has more sugar?________________________________________ Does the onion or potato contain more sugar?________________________________________ Spring 2006 2 Starch Test Starch turns bluish-black in the presence of iodine. Procedure: 1. Cut a very thin slice of onion and potato. Place each on a separate microscope slide. 2. Add one or two drops of iodine and a coverslip to each slide and observe them with the compound microscope. Describe what you see inside the potato cells (structures, which parts are stained, etc.): ______________________________________________________________________________ Describe what you see inside the onion cells (structures, which parts are stained, etc.): ______________________________________________________________________________ According to the results of the Benedict’s and starch tests, a potato plant stores most of its carbohydrates in the form of (starch or sugar?) _________________________, while onion plants store most of theirs in the form of (starch or sugar?) _____________________________. 3. Label two slides at one end with either the word “Green” or Ripe”. 4. Place a very small amount of mashed green banana and mashed ripe banana on the appropriate slide. Spread the sample on the slide with a toothpick so that it is very thin. 5. Add a drop of iodine and a coverslip. Observe the slides microscopically. Which banana has the most starch? ________________________________________________ Keeping in mind which banana has the most sugar and which has the most starch, describe the chemical changes that take place in a banana as it ripens: ______________________________ _____________________________________________________________________________ Wild bananas have seeds. How does becoming sweeter aid in the dispersal of these seeds? ______________________________________________________________________________ Name two other fruits or vegetables that follow the same pattern: _________________________ Lipids: Lipids are a diverse group of molecules that share one characteristic property: they do not dissolve in water. Lipids are an efficient form of energy storage. One gram of lipid contains twice as much energy as one gram of either carbohydrates or proteins. This form of condensed energy storage is especially useful to small active animals, such as birds. Lipids also play a Spring 2006 3 significant role in the structure of biological membranes. Cells, as we know them, would not exist without lipids. Despite popular belief, lipids (fats), are an important part of a balanced diet and can be found in oils, fried foods and nuts. Perform the following tests: 1. Add 10 mL of water to a test tube. 2. Add 10 drops of vegetable oil. Shake. Observe the dispersal of oil in the water followed by a rapid separation into two distinct layers. Which substance floats to the top? __________________________________________________ 3. Add to this tube a few drops of an emulsifier. An emulsifier breaks oil into small droplets and prevents them from separating into layers again. (This is how creamy salad dressings are made.) Shake the tube again. This mixture of oil and water is an emulsion. Describe the distribution of oil in the emulsion now compared to the previous test. ______________________________________________________________________________ 4. Put a drop of the emulsion (not the emulsifier) on a slide. Add a small drop of Sudan IV stain, mix, and add a coverslip. Examine microscopically. Which substance (water or oil) is stained by the Sudan IV? ______________________________ Proteins: Long chains of amino acids are joined together chemically by peptide bonds to form proteins. Proteins are much more than meat or muscle. They determine the shape and structure of living organisms and act as enzymes that promote the chemical reactions necessary for life. Twenty amino acids are used in the formation of proteins. Your body can manufacture only twelve while the other eight, the essential amino acids, must be provided by your diet. Foods high in the essential amino acids are fish, eggs, and beans. Amino Acids Most amino acids (except proline) will produce a violet color in the presence of the chemical ninhydrin. Proline produces a yellow color. Procedure: 1. Obtain a round piece of filter paper, being careful to handle it only by the edges. Label it as shown below with a pencil. Spring 2006 4 2. With a transfer pipette place a drop of the appropriate substance on the paper. Allow the spots to dry. In the “Thumbprint” circle press your thumb very firmly, trying to leave a heavy thumbprint. (This was one of the first methods used to try to reveal fingerprints.) 3. Spray these areas with 1% ninhydrin solution. Caution: Ninhydrin spray is to be used only in the fume hood. 4. Place the papers on the hot plate to dry. The color should develop in 15-20 minutes. Record your results in Table 2. Table 2. Results of amino acid test Sample Final Color Interpretation of Results Water Phenylalanine Proline Thumbprint Why does your thumbprint give a positive result? ______________________________________ Spring 2006 5 Proteins Biuret reagent changes color from 'sky blue' to pink or lavender in the presence of proteins, but not amino acids. Procedure: 1. Label four test tubes from 1-4. 2. With a graduated cylinder, add 10 mL Biuret reagent to each test tube. Caution: Biuret reagent is caustic. Do not get it on your skin or clothes. Wash off with water immediately. 3. Add an equal amount of the appropriate material listed in Table 3 to the appropriate test tube. 4. Record your results in Table 3. Table 3. Results of Biuret test for proteins Tube # Sample 1 Water 2 Egg white solution 3 Egg yolk solution 4 Phenylalanine Spring 2006 Final Color Interpretation 6 Testing Unknowns: Each group will be provided with an unknown food. The letter tells you the group of possible unknowns from which your sample came. Your food is one of the ones listed under that letter. A B C Soy flour Corn starch Ground coffee Glucose Honey Instant coffee Powdered milk Table sugar Enriched Flour Egg white D E Vermont Maid Syrup Glucose Maple syrup Table salt Potato starch Gelatin The compositions of these foods are given in Table 4*. Look on this table for simple sugar, starch, fat, and protein compositions of these foods. Low levels of particular molecules will probably not show up in these tests. Procedure: 1. Place a small sample of the unknown in each of several clean test tubes. Add the proper reagent for sugar to test tube #1. Which reagent will you use?______________________ 2. Use tubes 2, 3, and 4 to test for the other substances [starch (iodine), lipids (Sudan IV), and protein (Biuret)]. Do not add reagents to the entire original unknown. Look back to earlier parts of this lab to recall these procedures. Test only a small portion of solid unknowns and dissolve them in water when appropriate, such as for Benedict’s test. 3. Mix the reagent and unknown food thoroughly so that you don’t get a false negative reaction. 4. After each test, decide which, if any, substance on your list of possible unknowns was ruled out. For example, a positive test for simple sugar would rule out corn starch (starch must give a negative test). A negative test for simple sugar would rule out honey (glucose in honey must give a positive test). 5. Identify your unknown and enter it in Table 5. Spring 2006 7 Table 4. Composition of Foods Grams per 100 grams edible portion (or %). Amounts in parentheses cannot be detected by your tests. Corn Starch Egg White Egg Yolk Enriched Flour Gelatin (dry) Glucose Ground Coffee Honey Instant Coffee Maple Syrup Potato Starch Powdered Skim Milk Soy Flour Table Salt Table Sugar Vermont Maid Syrup Water % Protein % Fats % Carbohydrate 12 87.6 51.1 12 13.0 9 4.1 17.2 2.6 21.5 7.6 4 8 0.2 0.5 25 (0.3) 10.9 16.0 (10.5) 85.6 (0) (12.5) (0.3) (0) (0) (8.0) 35.8 41.2 (0) (0) (0) (trace) (trace) 30.6 (1.0) (0.1) (0) 15.4 (0) (0) (0) (0.8) (0.7) 12.1 (0) (0) (0) 87.6 (as starch) (0.8) (0.6) 76.1 (as starch) (0) 91 28.5 82.3 (as glucose + fructose) 35.0 (77) (only sucrose) 79.9 (as starch) 51.6 (as starch) 33.3 (0) (99.5) 74 (as glucose + fructose) Table 5. Results of Testing Unknowns. Letter of your unknown _____________. Test Performed Results Substance (s) Eliminated by This Test Your unknown substance is _______________________ Instructor’s Initials __________ Before you leave the lab . . . make sure that all the equipment you used is spotlessly clean for the next class. Be sure your work area has been washed clean. Do not throw any garbage down the sink. All solid wastes must go in the waste can. Modified slightly from Experiments in Biology (1988) by Carolyn Eberhard, published by Saunders College Publishing. **Watt, B. and A. Merrill, “Composition of Foods, Raw, Processed, Prepared.” Agricultural Handbook No. 8. Revised Dec. 1963. Sup. of Documents, U.S. Govt. Printing Office, Washington, DC. Spring 2006 8