srep05787-s1
advertisement

Supporting Information
SUBJECT AREAS: NANOSHEET • NICKEL HYDROXIDE • MICROWAVE
SYNTHESIS • SUPERCAPACITOR • ELECTROCHEMISTRY
Correspondence and requests for materials should be addressed to C. Cao (cbcao@bit.edu.cn)
Ultrathin Nickel Hydroxide and Oxide Nanosheets: Synthesis, Characterizations and
Excellent Supercapacitor Performances
Youqi Zhu1, Chuanbao Cao1, Shi Tao2, Wangsheng Chu2, Ziyu Wu2, Yadong Li3
1
Research Center of Materials Science, Beijing Institute of Technology, Beijing 100081, China
National Synchrotron Radiation Laboratory, University of Science and Technology of China,
Hefei 230029, China
2
3
Department of Chemistry, Tsinghua University, Beijing 100084, China
1
Figures Caption:
Figure S1. (a) Optical photographs of microwave reactor; (b), (c) the large-scale products of
α-Ni(OH)2 nanosheets (1.48 g) and NiO nanosheets (1.14 g) synthesized one times; (d) αNi(OH)2 nanosheets(green) and NiO nanosheets(black) dispersion in ethanol after ultrasonic
processing and standing for weeks.
Figure S2. (a) Thermogravimetric curve of α-Ni(OH)2 nanosheets in air with a heating rate of
10 ºC min−1, the thermodecomposing temperature is detected to be about 300 ºC; (b) XRD
patterns of α-Ni(OH)2 and the corresponding NiO nanosheets after heat treatment. As
demonstrated by XRD measurement, single crystalline phase of α-Ni(OH)2 is obtained, no
any other impurity or heterogeneous crystalline. Figure S2b shows that all the diffraction
peaks of Ni(OH)2 nanosheets are consistent with a hexagonal layered structure α2
3Ni(OH)2•2H2O with lattice parameters of a = b = 5.34 Å and c = 7.50 Å (JCPDS 22-0444).
The sole strong diffraction peak from (001) planes clearly reveals that the α-Ni(OH)2
nanosheets possess a highly preferred (001) orientation. The intensity ratio of I(001)/I(110) =
3.21 is much larger than that of the standard card (1.25), implying a 2D structure with
preferential direction along the ab plane. The calculated basal spacing is 7.49 Å for (001)
peak.
Figure S3. (a) EDS pattern record from the marked rectangular area of the inset for αNi(OH)2 nanosheets, showing that only Ni and O are detected; (b), (c), and (d) FESEM
images of the α-Ni(OH)2 nanosheets at different magnifications, revealing that The assynthesized α-Ni(OH)2 exhibit a geometrically graphene-like 2D structure. They are uniform,
no any other structure, such as nanoparticle, can be observed. The nanosheets display a typical
crinkly and rippled structure. These surface wrinkles are believed to accommodate the
excessive surface energy caused by the ultrathin thickness for improving structural stability
and flexibility. The lateral size is large up to several micrometers with all edges curling.
Figure S3d further reveals that the ultrathin α-Ni(OH)2 nanosheets are constituted by ultrafine
nanoplates (or nanocrystals), which reflect the intrinsically structural property of this kind of
3
layered compound. Despite some wrinkles, the surface of these nanosheets is also rough due
to the presented grain boundaries, which can further enhance the surface area.
Figure S4. HRTEM image of (a) a vertical and (b) a planar of α-Ni(OH)2 nanosheets; (c)
SAED pattern and (d) enlarged HRTEM image from (b). Figure S4b shows the horizontal
unfold nanosheets. Figure S4c shows the hexagonal symmetry and single-crystalline nature.
There are two marked lattice fringes, corresponding to the {200} and {204} planes,
respectively. Figure S4d shows that all the α-Ni(OH)2 nanocrystals share the same lattice
fringe and crystallographic orientation, indicating their the same behavior in the arrangement.
The marked lattice spacing is calculated to be 0.262 nm, corresponding to the (110) plane
with [001] zone axis. The α-Ni(OH)2 nanosheets possess a microscopic orientation along c
axis, i.e. (001) crystal plane that exposed outside.
Figure S5. (a) XPS spectrum, and (b) the deconvoluted Ni 2p of as-prepared α-Ni(OH)2
nanosheets. The XPS analysis is further carried out to determine the composition and the
surface electronic state of the as-synthesized α-Ni(OH)2 nanosheets (Figure S5). It can be
4
clearly found that the survey spectrum of the α-Ni(OH)2 nanosheets mainly shows oxygen and
nickel species (Figure S5a), no any other species are detected, which is in accordance with
EDS analysis. As shown in Figure S5b, the appearance of two major peaks cantered at 873.5
and 855.9 eV can be assigned to Ni 2p1/2 and Ni 2p3/2, respectively, with a spin-energy
separation of 17.6 eV, which is the characteristic of a Ni(OH)2 phase. This result together
with the XRD and EDS analysis demonstrates advantages of the microwave-assisted liquid
phase synthesis in producing high-purity samples. Additionally, it is worth noting that there
are some extra lines marked as satellite peaks around the expected Ni 2p1/2 and Ni 2p3/2
signals in the Ni 2p region.
Figure S6. (a), (b) N2 adsorption-desorption isotherm of α-Ni(OH)2 and NiO nanosheets. The
inset shows the pore size distributions. The isotherm belongs to type IV and exhibits an
obvious hysteresis loop, indicating a typical mesoporous structure. The most probable pore
size of α-Ni(OH)2 nanosheets is 2.43 nm using the Barrett–Joyner–Halenda model. This value
roughly corresponds to the inter-particle space between ultrafine nanocrystals. The pores of
NiO nanosheet could be created by the evacuation of gaseous contents. The most probable
pore size of NiO nanosheet is 2.73 nm. The pores in a wide range of pore size should stem
from the restack of the sheets.
5
Figure S7. FESEM images of non-layered NiO nanosheets synthesized with different
microwave irradiating time: (a) 3 minutes, (b) 5 minutes, (c) 15 minutes, and (d) 30 minutes.
Other conditions are unchanged.
Figure S8. FESEM images of NiO nanomaterials synthesized in different reactive medium:
(a), (b) water/ethylene glycol (1:14 by volume), and (c), (d) absolute ethylene glycol. Other
conditions are unchanged.
6
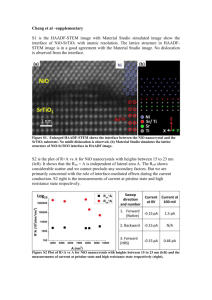
Figure S9. Electrochemical characterization of non-layered NiO nanosheets: (a) discharge
curves at different current densities; (b) specific capacitance as a function of the current
densities calculated from the corresponding discharge curve; (c) average specific capacitance
versus cycle number at a current density of 4 A g−1, the inset shows the galvanostatic chargedischarge curves; (d) Nyquist plots of non-layered NiO nanosheets before and after chargedischarge cycling at a current density of 4 A g−1, (inset) equivalent fitting circuit and
impedance at high frequency region, where the Rs, Rf, Cdl, W, and Cf represent the internal
resistance, charge-transfer resistance, double layer capacitance, Warburg impedance, and
Faradaic pseudocapacitance, respectively.
Electrochemical performances analysis in detail of non-layered NiO nanosheets: The distinct
plateau in every discharge curve (Figure S9a) also corresponds to the Faradaic discharge
process. The non-linear curve shape verifies the pseudocapacitive charge storage mechanism.
As expected, the ultrathin NiO nanosheets deliver an ultrahigh specific capacitance of 2236 F
g−1 at 0.5 A g−1, which is the highest value so far as known among the nickel oxide-based
electrode materials. Because pseudocapacitance is mainly from a region near the surface, the
practical specific capacitances for a wide range of unique NiO nanostructures are still far
below the theoretical value (2584 F g−1).1,2 However, for our NiO nanosheets, nearly all
pseudocapacitive materials are exposed on surface for the Faradaic reactions, allowing for a
7
maximum active material utilization. As the current density increased, the ultrahigh specific
capacitance is still maintained, which are 1966, 1820, 1576, 1452, and 1392 F g−1 at 1, 2, 4, 6,
and 12 A g−1, respectively (Figure S9b), indicating excellent rate capability. All the obtained
specific capacitances are higher than the results in previous reports checked at the similar
current density (Table S1). The ultrathin NiO nanosheets also exhibit an excellent cycling
stability with good retention of capacitance of 1562 F g−1 at 4 A g−1 after 2000 cycles,
equivalent to 99.1% of the initial capacitance (Figure S9c). The insert further indicates that
the fast charge-discharge process is highly reversible. The excellent properties could be
attributed to the unique ultrathin 2D nanostructure of NiO electrode materials, which can
provide short ion and electron diffusion path distances, large electrochemical active sites and
electrode-electrolyte interface. In the desirable architecture, a maximum active material
utilization and favorable reaction kinetics are guaranteed. As demonstrated by impedance
analysis (Figure S9d), the ultrathin NiO nanosheets show a low charge-transfer resistance (Rf).
After 1000 cycles, the Rf is not significantly altered, indicative of the best electrical
conductivity and activity with favorable reaction kinetics. The steeper line in the low
frequency range indicates an ideal capacitor.
Table S1. A comparison of various relatively good results of nickel oxide supercapacitors
materials
NiO hollow nanosphere
NiO nanosheet hollow spheres
Porous NiO nanosheets
3D nanoporous NiO film
NiO/Ni Nanocomposites
NiO Nanobelts
Mesoporous NiO nanowire networks
Porous NiO nanospindles
Au–NiO composite
NiO nanoflower
Specific capacitance
current density
(F g−1) [a]
(A g−1)
770
511
1025
1790
905
1126
555
1462
765
480
2
2
3
2.5
1
2
2
1
2
0.5
Ref.
3
4
5
6
7
1
8
9
10
11
[a] the specific capacitance were calculated by galvanostatic discharge curves.
References
8
[1] B. Wang, J. S. Chen, Z. Wang, S. Madhavi, X. W. Lou, Adv. Energy Mater. 2012, 2,
1188.
[2] B. Qu, L. Hu, Y. Chen, C. Li, Q. Li, Y. Wang, W. Wei, L. Chen, T. Wang, J. Mater.
Chem. A 2013, 1, 7023.
[3] C. Y. Cao, W. Guo, Z. M. Cui, W. G. Song, W. Cai, J. Mater. Chem. 2011, 21, 3204.
[4] S. Ding, T. Zhu, J. S. Chen, Z. Wang, C. Yuan, X. W. Lou, J. Mater. Chem. 2011, 21,
6602.
[5] X. Sun, G. Wang, J. Y. Hwang, J. Lian, J. Mater. Chem. 2011, 21, 16581.
[6] K. Liang, X. Tang, W. Hu, J. Mater. Chem. 2012, 22, 11062.
[7] Q. Lu, M. W. Lattanzi, Y. Chen, X. Kou, W. Li, X. Fan, K. M. Unruh, J. G. Chen, J. Q.
Xiao, Angew. Chem. 2011, 123, 6979.
[8] X. Li, S. Xiong, J. Li, J. Bai, Y. Qian, J. Mater. Chem. 2012, 22, 14276.
[9] H. Pang, B. Zhang, J. Du, J. Chen, J. Zhang, S. Li, RSC Adv. 2012, 2, 2257.
[10] B. Qu, L. Hu, Y. Chen, C. Li, Q. Li, Y. Wang, W. Wei, L. Chen, T. Wang, J. Mater.
Chem. A, 2013, 1, 7023.
[11] S. I. Kim, J. S. Lee, H. J. Ahn, H. K. Song, J. H. Jang, ACS Appl. Mater. Interfaces, 2013,
5, 1596.
9