SimAssign 02
advertisement
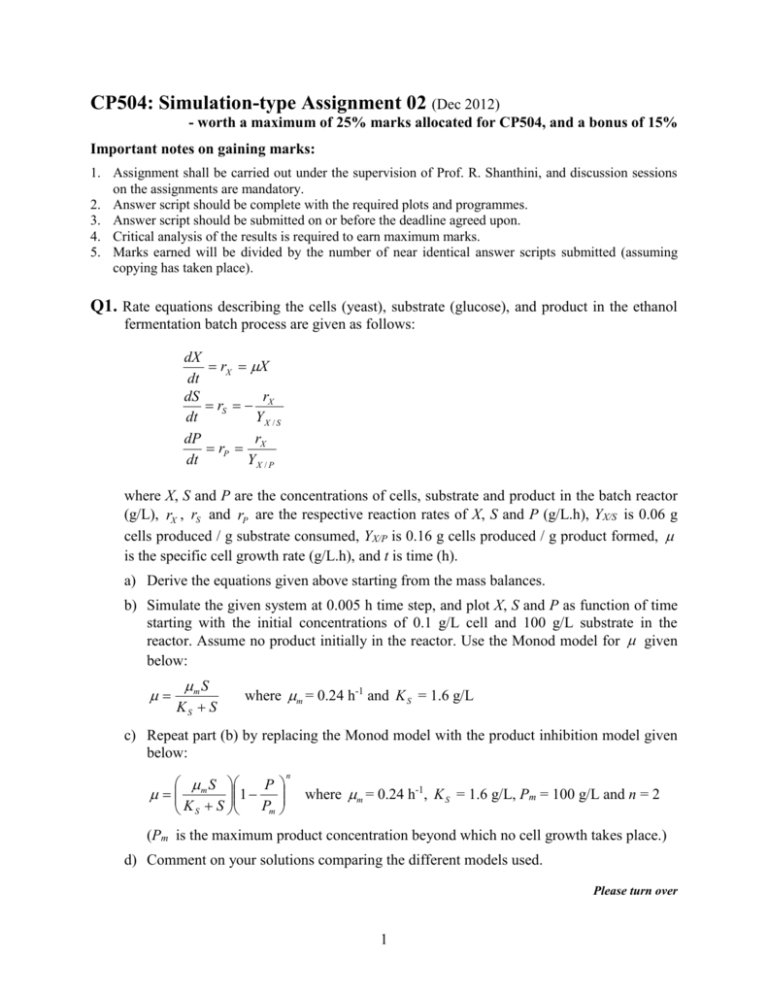
CP504: Simulation-type Assignment 02 (Dec 2012) - worth a maximum of 25% marks allocated for CP504, and a bonus of 15% Important notes on gaining marks: 1. Assignment shall be carried out under the supervision of Prof. R. Shanthini, and discussion sessions on the assignments are mandatory. 2. Answer script should be complete with the required plots and programmes. 3. Answer script should be submitted on or before the deadline agreed upon. 4. Critical analysis of the results is required to earn maximum marks. 5. Marks earned will be divided by the number of near identical answer scripts submitted (assuming copying has taken place). Q1. Rate equations describing the cells (yeast), substrate (glucose), and product in the ethanol fermentation batch process are given as follows: dX rX X dt dS r rS X dt YX / S r dP rP X dt YX / P where X, S and P are the concentrations of cells, substrate and product in the batch reactor (g/L), rX , rS and rP are the respective reaction rates of X, S and P (g/L.h), YX/S is 0.06 g cells produced / g substrate consumed, YX/P is 0.16 g cells produced / g product formed, is the specific cell growth rate (g/L.h), and t is time (h). a) Derive the equations given above starting from the mass balances. b) Simulate the given system at 0.005 h time step, and plot X, S and P as function of time starting with the initial concentrations of 0.1 g/L cell and 100 g/L substrate in the reactor. Assume no product initially in the reactor. Use the Monod model for given below: m S KS S where m = 0.24 h-1 and K S = 1.6 g/L c) Repeat part (b) by replacing the Monod model with the product inhibition model given below: S P m 1 Pm K S S n where m = 0.24 h-1, K S = 1.6 g/L, Pm = 100 g/L and n = 2 (Pm is the maximum product concentration beyond which no cell growth takes place.) d) Comment on your solutions comparing the different models used. Please turn over 1 Q2. Rate equations describing the cells (X), substrate (S), and product (P) in a continuous stirred tank fermenter (CSTF) operated at unsteady state are given as follows: dX rX DX where rX X dt dP rP DP where rP X dt dS YS / X rX YS / P rP D( Sin S ) dt where X, S and P are the concentrations of cells, substrate and product in the CSTF (g/L), rX and rP are the respective reaction rates of X and P (g/L.h), YS/X is 5.1 g substrate consumed / g cell produced, YS/P is 1.5 g substrate consumed / g product formed, is the specific cell growth rate (g/L.h), is the specific product synthesis rate (g/L.h), D is the dilution rate (0.15 per h), Sin is the substrate concentration in the feed (95 g/L), and t is time (h). a) Derive the equations given above starting from the mass balances. b) Simulate the given system to find out the steady state values of X, S and P starting with different initial concentrations of X. Assume no product or substrate in the reactor initially. Use the following models to describe and : S 2 K S1 S S / K I m m K P P 1 (substrate-product inhibition) K P P Pm S KS2 S where m (= 0.3 h-1) is the maximum specific cell growth rate, m (= 0.11 h-1) is the maximum specific product synthesis rate, K S 1 (= 0.26 g/L) and K S 2 (= 9.5 g/L) are the saturation constants, K I (= 297 g/L), K P (= 8 g/L) and Pm (= 85 g/L, which is the maximum product concentration beyond which no cell growth takes place) are the inhibition constants. c) Comment on your solutions Reference for Q1: Problem 6.4 in Lee JM. 1992. Biochemical Engineering, Prentice-Hall. Reference for Q2: Problem 5.15 in Jana, A.K. 2008. Chemical Process Modelling and Computer Simulation. Prentice-Hall of India (Pvt) Ltd. 2