Miniprep protocol
advertisement

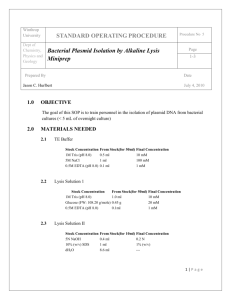
Alkaline Lysis Miniprep 1 Alkaline Lysis Miniprep Protocol Day 1 1. 2. 3. 4. Write procedure in notebook. To clean sterile 18 ml test tubes add 3 ml LB + ampicillin1 (or appropriate antibiotic). Inoculate each tube with one isolated colony (do not forget the negative control2). Incubate tubes in water bath shaker at 37 °C, overnight. Day 2 5. Make fresh Lysis solution3 6. Pour overnights (after shaking tubes to resuspend cells) into a 1.7 ml microcentrifuge tube, which has been appropriately labeled. Centrifuge the microcentrifuge tubes to pellet the cells4. Aspirate out the supernatant carefully without disturbing (touching) the cell pellet. Repeat the above steps, in the same tube, with the remaining overnight culture. 7. Add 300 L Resuspension solution5 to the pellet and completely resuspend the cells by vortexing. 8. Add 300L of Lysis solution, mix by gently inverting 4-5 times. 9. Add 300L of Neutralization solution6, mix by gently inverting 4-5 times. 10. Centrifuge microcentrifuge tubes for 10 minutes at appropriate speed4, 7,8. 11. Transfer supernatant to new microcentrifuge tubes and recentrifuge for 10 minutes at appropriate speed4. (IF WHITE PRECIPITATE IS STILL PRESENT IN SUPERNATANT, TRANSFER SUPERNATANT TO NEW TUBE AND RECENTRIFUGE) 12. Transfer supernatant to new microcentrifuge tubes containing 0.6 volumes (this is 540 l for this protocol) of isopropanol, mix by gently inverting 4-5 times. Centrifuge for 10 minutes at appropriate speed4, 8. 13. Aspirate out the supernatant (carefully avoiding the side of the tube where the DNA is located)8. 14. Add 500L of 70% ethanol9, centrifuge for 5 minutes at appropriate speed4. 15. Aspirate out the supernatant (carefully avoiding the side of the tube where the DNA is located)8 16. Add 30 l of sterile diH2O to microcentrifuge tube, vortex briefly to resuspend DNA. 17. SpeedVac for 5 minutes to remove remaining ethanol10. 1. 2. 3. Stock concentration of ampicillin is 100 mg/ml. The final concentration in the LB broth should be 100 g/ml. (To calculate the volume that needs to be added, look at “Calculating Concentrations” page). BE SURE SOLUTION IS COMPLETELY MELTED AND MIXED BEFORE ADDING. The negative control is a test tube containing 3 ml of LB + ampicillin, inoculated with a sterile inoculating stick. The Lysis solution is composed of 1% SDS and 0.2N NaOH (you will need 0.3 ml of the Lysis solution for each sample. Look at “Calculating Concentrations” page for instruction on calculating volumes). The Lysis solution needs to be made fresh each time you miniprep. When making lysis solution, mix thoroughly using a pippet tip, to dissolve any precipitate. Avoid introducing bubbles in solution. Alkaline Lysis Miniprep 2 4. If using the Eppendorf 5415C centrifuge, centrifuge at 14,000 rpm for 1 minute. If using the Eppendorf 5417 centrifuge, centrifuge at 11,000 rpm for 1 minute. 5. The Resuspension solution is composed of 50 mM Tris-Cl (pH 7.5) and 10 mM EDTA (pH 8.0) (you will need 0.3 ml of the Resuspension solution for each sample. Look at “Calculating Concentrations” page for instruction on calculating volumes). Before using the Resuspension solution, you must add RNase A (DNase free) to a final concentration of 100 g/ml (stock concentration is 100 mg/ml). 6. The Neutralization solution is 1.32 M potassium acetate (C2H3O2K) at pH 4.8 7. When centrifuging, be sure to balance the rotor. The rotor can be balanced by multiples of 2 or 3 (ask co-workers for clarification). A set of balanced tubes must have equal weight. 8. When placing the microcentrifuge tubes in the rotor, keep tube hinges facing outward. This will cause the precipitated material to be centrifuged to that location. This is important when trying to identify the location of precipitated DNA, which is clear. 9. The 70% ethanol is composed of 100% ethanol and diH2O (Look at “Calculating Concentrations” page for instruction on calculating volumes). 10. Ethanol will inhibit subsequent experiments. Therefore, it is essential to remove all remaining ethanol from the miniprep.