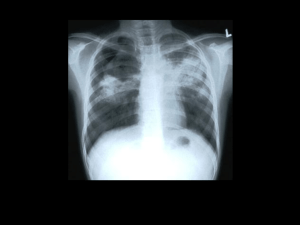
Tuberculosis - WHO archives
advertisement

Chapter 6.8: Tuberculosis Priority Medicines for Europe and the World "A Public Health Approach to Innovation" Background Paper Tuberculosis By Dr Mary Moran 7 October 2004 6.8-1 Chapter 6.8: Tuberculosis Table of Contents Summary ............................................................................................................................................... 3 Introduction What is the Size and Nature of the TB buRden?................................................................................ 4 What is the TB Control Strategy? ......................................................................................................... 6 Why Does the Disease Burden Persist?............................................................................................... 8 Research into Pharmaceutical Interventions Past and Present: What Can be Learnt? ........ 10 Diagnostics ............................................................................................................................................ 10 Drugs ..................................................................................................................................................... 11 Vaccines ................................................................................................................................................. 18 Existing Resource Flows for TB R&D............................................................................................ 20 Basic research ....................................................................................................................................... 20 Diagnostics ............................................................................................................................................ 20 Drugs ..................................................................................................................................................... 20 The Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) .................... 22 Vaccines ................................................................................................................................................. 24 Resource flow lessons.......................................................................................................................... 25 What is the Current Pipeline? ......................................................................................................... 25 TB Diagnostics ...................................................................................................................................... 25 TB Drugs ............................................................................................................................................... 26 TB Vaccines ........................................................................................................................................... 26 Opportunities for Research into New Pharmaceutical Interventions ..................................... 27 Are there research gaps that could be filled affordably, have a significant impact and be achieved in a) 5 years or b) the longer term? Which of these are pharmaceutical research gaps?..................................................................................................................................................... 30 Basic research ....................................................................................................................................... 30 Diagnostics ............................................................................................................................................ 31 Drugs ..................................................................................................................................................... 32 Vaccines ................................................................................................................................................. 37 Conclusion .......................................................................................................................................... 38 References ........................................................................................................................................... 40 Annexes Appendices 6.8-2 Chapter 6.8: Tuberculosis Summary Tuberculosis is a major and growing threat. The expanded European Union now has a substantial and increasing TB burden of more than 50,000 cases per year, around 10% of whom have TB which is already resistant to one or more of our existing drugs. And globally, TB control is now threatened by the upsurge in HIV-TB co-infected patients, who are straining current TB tools and DOTS approaches to the limit. Controlling TB with our existing tools is a cumbersome, expensive and sometimes unsuccessful task. There are no cheap, rapid, reliable diagnostics for TB screening or MDRTB diagnosis, and our first-line TB diagnostic test picks up only around 50% of patients with active TB. Drug therapies are resource-intensive and expensive, particularly in European settings, requiring 6-8 months of therapy with up to one-hundred observed doses. Therapies for MDR-TB are even longer, up to 2 years, and have high failure rates. And there is no reliable vaccine to prevent TB in adults. Although new tools and approaches are being developed in all areas – including basic research, drugs, diagnostics and vaccines - progress is being delayed by lack of targeted funding and support, in particular from the EU which now provides less than 5% of global funding for development of new TB tools. The U.S. is driving the TB R&D agenda, particularly for new drugs, with the preponderance of research and development now being funded by the U.S. government and U.S. philanthropists, and with U.S.-based industry and academic groups being the main collaborators (and beneficiaries of R&D contracts). The landscape of R&D for these new tools has also changed dramatically, with the major pharmaceutical industry no longer playing a lead role. Development of new TB drugs, diagnostics and vaccines is now almost entirely driven by public-private partnerships, funded by philanthropists; while industry participation tends to be from smaller companies, such as biotech firms and contract research organisations, rather than large multinational companies. Those large companies who are involved – for instance in developing vaccine adjuvants or working on the discovery stage of TB drugs – are almost all EU-based. Current EU funding for R&D of new TB tools does not always reflect these new epidemiological and pharmaceutical realities. EU funding is insufficient (around $8.5 million per year under the Fifth Framework Programme) and is not well targeted at the PPPs and smaller industry and academic groups who are now most active in TB research and development. This paper suggests a new EU funding model which would support European industry and academic research while ensuring it is closely focussed on optimal health outcomes for TB patients and the governments who provide their care. 6.8-3 Chapter 6.8: Tuberculosis Introduction TB is an infectious disease, caused by M. tuberculosis. Although it most commonly presents as pulmonary TB (80% of patients), up to 1 in 5 patients present with extra-pulmonary manifestations, including miliary TB, bone and joint TB and TB meningitis.1 TB normally progresses slowly from the latent stage (infection without active disease) to active TB disease, except in HIV co-infected patients where progression can be rapid and fatal. What is the Size and Nature of the TB burden? One in three people globally is infected with TB (latent TB), although only a small proportion of these progress to clinical disease (active TB).2 In 2001, an estimated 8.5 million cases of TB disease occurred globally, with just under half of these being diagnosed (3.8 million cases) and around three-quarters of diagnosed cases being cured. a3 The bulk of TB is concentrated in 22 high-burden countries, all in the developing world, while multi-drug resistant TB (MDR-TB) is focussed in “hot spots” such as Latvia, Estonia, parts of the Former Soviet Union and several Chinese provinces.4 TB now ranks as the ninth most significant burden of disease globally, representing 2.4% of all DALYs lost – as a comparison, HIV/AIDS is responsible for 5.8% of lost DALYs and malaria for 3.0%.5 However, although the extensive burden of TB is a concern, a greater cause of disquiet is the rapid growth of TB in some regions, leading to an overall increase in TB notifications of around 0.4% per year (see Fig 1). Fig.1: Global trends in TB case notification6 Global trends in case notifications 170 Standardized case notification rate 160 150 140 130 120 110 100 90 1980 1985 1990 1995 2000 This overall trend hides important disparities in TB control. Within Western Europe there are several countries with increasing TB rates (including the UK, Denmark and Norway) and TB burdens in Eastern Europe and Africa are growing rapidly.7 However, many regions, including the Eastern Mediterranean; South East Asia; Western Europe; and India and China (which together make up 40% of the global TB burden) have stable or declining TB notification rates, consistent with improved socio-economic conditions and more rigorous implementation of TB treatment programmes. Global cure rates are 49% (non-DOTS programmes) and 74% (DOTS programmes). If cure plus completion of treatment are measured, these figures are 67% and 82% respectively. (The same reference applies.) a 6.8-4 Chapter 6.8: Tuberculosis Fig. 2 Fig. 38 The rise and rise of TB in Africa, linked to HIV The epidemiology of TB in Europe 400 Former Soviet Standardized case notificaiton rate 300 Sta notificat nd 250 ion rate ard ize 200 d ca 150 se 100 Central Europe Established Markets 350 300 250 200 150 100 50 50 0 0 1980 1980 1985 1990 1995 1985 1990 1995 2000 2000 The rise of TB in Eastern European countries is linked to declining social conditions and the breakdown of health-care systems (including TB programmes) during the transition to market-based economies. The impact of this socio-economic decline was compounded by a lack of political commitment to DOTS, with many Former Soviet Union (FSU) countries continuing to use inappropriate TB control strategies, such as non-DOTS protocols and unproven revaccination programmes.9 As a result, these countries – including many EU accession countries – have high and rapidly increasing rates of both TB and MDR-TB. (See Annex 6.8.1a-c) Globally, the rise of TB, particularly in Africa, is largely due to the unchecked HIV/AIDS pandemic. This is due to the direct role played by HIV immunosuppression in activation of latent TB; the sheer number of new TB patients spreading the disease; the poorer performance of TB tools in HIV+ immunosuppressed patients; and the impact of HIV on health systems, health workers and the general economy. Three factors are likely to impact on the future TB burden in Europe. The accession of Central European and Former Soviet Union countries means the expanded EU will soon face a substantially increased burden of TB and MDR-TB. Although much of this will remain within the borders of the accession countries, migration means that the relatively TB-naïve health systems of Western Europe will also be exposed to increased TB, including potentially untreatable drug-resistant strains of TB (at least one-third of MDR-TB patients do not respond to existing drugs). 10 Around 20,000 TB cases were notified in the first-rank accession countries in 2001, with TB incidence rates as high as 75-85 per 100,000 in Latvia and Lithuania - by comparison, France and Germany have incidence rates around 10 per 100,000. (See Annex 6.8.2) Latvia and Estonia also had the dubious honour of being in the Top 5 for both MDR-TB and poly-drug resistant TB (MDR was a disturbing 18% in Estonia and 12% in Latvia).11 Central and Eastern Europe and the Former Soviet Union (CEE/FSU) currently combine some of the world’s highest MDR-TB rates with the world’s fastest growing HIV infection rates. In 1995, the number of HIV infections in the region was estimated at below 30,000. By end 1999, this had climbed to 420,000, reaching 700,000 in 2000.12 The course of the TB-HIV epidemic in Sub-Saharan Africa (where MDR-TB rates are low) is a salutary lesson for the CEE/FSU region, but one which is not being adequately addressed. 6.8-5 Chapter 6.8: Tuberculosis In the longer term, economic growth in the accession countries is highly likely to reduce their burden of both TB and MDR-TB. Globally, the two factors most likely to influence the TB burden are: DOTS expansion, which delivers increased TB detection and cure rates in areas of DOTS coverage. There has been a renewed push for DOTS expansion, with rapidly increased coverage in four of the top five high-incidence countries (India 45%; China 68%; Indonesia 98% and Bangladesh 95%).13 Results from Vietnam, Peru and early results from China show that DOTS can reduce the TB burden, however we will need to substantially improve case-finding tools and strategies, which currently find a minority of TB patients even with 100% DOTS coverage. The HIV/AIDS pandemic, particularly in Sub-Saharan Africa, but increasingly in SouthEast Asia, India and China. Country data show that HIV-TB has grown from 4% of the global TB burden in 1995 to 12% in 2000, with Africa now representing 20% of all TB cases.14 Without new TB tools and additional approaches, new AIDS epidemics in other areas of the world will be catastrophic for TB control. Scaling up of joint TB/HIV activities will be required. What is the TB Control Strategy? The fundamental TB control strategy, recommended for all epidemiological settings, is DOTS (Directly Observed Treatment, Short-course). Its five key elements are:15 Political commitment and resources to address TB Uninterrupted supply of the four to six most effective anti-TB drugs A standardised recording and reporting system to enable outcome assessment of patients and TB programmes Access to sputum microscopy to detect patients with smear-positive pulmonary TB Standardised 6-8 month drug therapy, with directly observed treatment for at least the first 2 months. In high HIV-TB settings, DOTS is supplemented by scaling up of 12 collaborative TB/HIV activities defined in the interim Policy on Collaborative TB/HIV Activities (ref: WHO/HTM/TB/2004.330; WHO/HTM/HIV/2004.1. DOTS-Plus for the treatment of MDR-TB (including access to cheaper drugs) is now also being piloted by WHO in a limited number of settings.16 Finally, wider use of anti-retroviral therapy in developing countries is seen as an important adjunct to TB treatment. The effectiveness of DOTS and its supplementary programmes varies depending on the epidemiological setting. Epidemiological modelling suggest that, in normal settings, DOTS can deliver a 6-7% annual TB decrease if coverage is 100% and if TB programmes can detect 70% of smear-positive patients and cure 85% of these – the so-called 70/85 targets. These figures are premised on passive case-finding, rather than active TB screening or contacttracing, as is used in most Western countries. The passive case-finding approach has been partly dictated by the absence of cheap, reliable screening tests to detect either latent or smear-negative TB, with most developing countries unable to afford the more sophisticated 6.8-6 Chapter 6.8: Tuberculosis diagnostics available in the West. Modelling also suggests that achieving DOTS targets would have averted 3.4 million additional TB deaths between 2001 and 2005, and allowed a total of 12.8 million people to be treated for TB.17 However, real-life results of DOTS are more mixed. DOTS clearly delivers higher detection and cure rates of smear positive new pulmonary cases than non-DOTS approaches.b But DOTS implementation has been slow and, even when implemented, most developing countries are struggling to reach the global 70/85 targets, in particular the 70% case-finding target.c Only two of the 22 high-burden countries (Vietnam and Peru) have achieved the 70/85 targets; and case-finding rates in the major-burden TB countries (India, China, Indonesia, Nigeria, Bangladesh) are still below 35%.18 Importantly, there are two settings where the DOTS strategy alone does not appear to be adequate without additional activities. The first is high-prevalence HIV settings, where DOTS can slow the increase in TB but “on its own ... is unlikely to reverse the upward trend”. 19 This view is confirmed by epidemiological modelling for Sub-Saharan Africa which suggests that, even if full DOTS implementation to the 70/85 targets could be achieved (in itself problematic), growth in TB will only be slowed from 10% to 7% per year.20 Collaborative TB/HIV activities will be required in these circumstances. The second area is MDR-TB. DOTS reduces generation of new MDR-TB cases, but management of existing MDR-TB patients is beyond the capacity and finances of most developing country DOTS programmes. In the absence of suitable tests, most MDR-TB patients are diagnosed on the basis of two failed courses of DOTS (15 months therapy) – during which time they remain infective with drug-resistant TB strains. Once diagnosed, the vast majority receive no further treatment, since MDR-TB treatment is around 1,400 times more expensive than TB treatment, with the drugs alone costing up to $8,000-13,000 per patient. DOTS-Plus, which offers subsidised drugs, currently covers only around 2,500 of the estimated 300,000-400,000 patients with MDR-TB, although additional patients are treated outside DOTS-Plus in wealthier countries such as South Africa or Latvia.21 Delays in diagnosis, lack of treatment, and relatively low cure rates in patients who are treated, all mean that MDR incidence is not being reduced as quickly as possible. The DOTS Plus strategy is required in these circumstances. DOTS averages 60% detection rates compared to 36% under non-DOTS programmes, and 77% cure-rates compared to 52% for non-DOTS. (WHO Global TB Control Report 2003, pp19-23) c After a decade of DOTS, 71% of TB patients globally are still treated outside DOTS programmes and slightly less than half of the 22 high-burden TB countries have achieved country-wide DOTS coverage of 95% or greater, while all top five high-incidence TB countries continue to have case-finding rates of around 35% or less. b 6.8-7 Chapter 6.8: Tuberculosis Why Does the Disease Burden Persist? Obstacles to improved TB control fall into two main categories. The first are obstacles related to DOTS itself; while the second are factors relating to developing country health care more generally. The main DOTS-related obstacles are: 1. Technical limitations of existing DOTS tools. WHO and CDC staff noted in 2001 that “as DOTS coverage has expanded, it has become apparent that the performance of existing tools for TB diagnosis and treatment limits more efficient implementation of the strategy”. 22 Key limitations are: Lack of cheap, quick and reliable tests for: - Screening for latent TB (which would allow preventative therapy to be given before infectious or active TB supervene); - TB diagnosis. Our fundamental TB diagnostic test, AFB microscopy, has an overall detection rate of only 50% (up to 75% in pulmonary cases) and is cumbersome, requiring trained microscopists and repeat patient visits. There is no suitable test for detection of TB in paediatric, extra-pulmonary or smear-negative patients in poor settings, many of whom are HIV-positive; - MDR-TB diagnosis. Current diagnosis relies on manual culture, which is cheap but extremely time-consuming (results take 6-9 weeks), and must be performed in a National Reference Laboratory or its equivalent. The scarcity of such laboratories means culture and drug-sensitivity testing (DST) are barely used in most developing countries, who instead rely on lengthy treatment trials. (See Annex 6.8.3) Lack of short-course drugs in long-acting formulations. Current TB drugs are cheap and can deliver cure rates of 85% or more. However they must be given daily or three-times weekly for 6-8 months (with observation of each dose for the first 2 months; and for all doses in rifampicin-containing regimens) Lack of an effective adult TB vaccine Lack of effective drugs for MDR-TB. Current drugs are described by WHO as “inherently more toxic and less effective than first-line drugs”23; they require treatment courses of 20-24 months; are prohibitively priced at up to US$8,000-13,000 per patient; and typically cure only around half to two-thirds of patients.24 2. DOTS “cumbersome, labour intensive and expensive” system of administration (as it was described by staff at WHO and the CDC) makes it difficult for developing countries to implement DOTS or to reach the global 70/85 targets. 25 The programme measures needed to overcome the technical weaknesses of existing TB tools (for example, repeat testing, lengthy treatment courses and frequent direct observation of patient therapy) now significantly outweigh the cost of the drugs themselves – indeed, they commonly represent around 90% of the cost of DOTS. 6.8-8 Chapter 6.8: Tuberculosis Table 1: Estimated public sector health system costs per treated case of infectious TB, excluding MDR-TB (US$)26 Country United Kingdom India China Uganda Thailand Russia Total $9,029 $57-$201 $61-$75 $430-$541 $219-$280 $1,115-$1,395 Drugs $200 $7 $18 $32 $43 $83 The costs of MDR-TB treatment are even more prohibitive, reaching as high as US $250,000 per patient in Western settings. Although the drugs are very expensive, the chief costs are incurred by hospitalisation, direct observation and monitoring during the 2-year treatment course needed with these old second-line drugs. We note that Latvia alone needs to spend Euro 6.3 million to manage 600 MDR-TB patients.27 This is more than twice the sum of money dedicated annually by the EC to development of shorter, more effective treatments for TB and MDR-TB. 3. The 70% case detection target may not be realistic using current diagnostic tools and passive case-finding approaches, with recent WHO research suggesting TB case-finding may plateau at 40-50% of smear-positive (infectious) patients.28 The second group of obstacles are factors relating to developing country health care more generally, and which apply to other diseases as well as TB: 1. The 22 high-burden TB countries identified five main country-level constraints to reaching the DOTS targets, including: lack of human resources; impact of health system restructuring, in particular decentralisation; poor health system infrastructure, organisation and management; private sector non-compliance with DOTS recommendations; lack of political commitment in some developing countries. Additional factors included poor drug supply systems; and poor physical access to treatment centres, especially for remote patients. 29 2. A funding gap. In 2003, the WHO reported the known country-level gap in TB funding at $219 million; the probable additional country-level gap at $838 million; and the gap in international technical assistance at $115 million (all figures for the 22 high-burden countries only).30 These gaps are likely to widen, particularly in SubSaharan Africa, as increased numbers of HIV patients are detected (many co-infected with TB) in response to current international initiatives. These include the WHO 3 x 5 initiative; the US $15 billion Bush grant; the work of the GFATM and Clinton Foundation; and WHO’s policy on TB/HIV collaborative activities. 3. Patient factors, including TB stigma and reliance on alternative healthcare providers. 6.8-9 Chapter 6.8: Tuberculosis Research into Pharmaceutical Interventions Past and Present: What Can be Learnt? Diagnostics Research into new TB diagnostics has been increasing in the past 10-15 years, with research and development toward new tests for smear-negative TB, TB screening, and rapid detection of MDR-TB; as well as tests to improve or replace sputum-based AFB for smear-positive patients. Much of the commercial R&D for TB has been undertaken by small and medium sized biotechnology companies taking advantage of the advanced state of diagnostics science, the lower development costs than for pharmaceuticals, and the presence of a modest Western market. Over 50 groups in 18 countries are developing or already marketing new TB tests, though of the largest 10 diagnostic companies, only 3 have any products for this disease. Academic research supporting these TB diagnostics development activities is carried out by a wide range of institutions, with support coming primarily from national research institutes or other public funds. Although multiple new tests have been developed for all patient categories, review of these reveals a number of weaknesses. The lack of regulatory oversight for diagnostics in developing countries means that clinical evaluations are often poor and advertised test specifications often overstate their efficacy. Smaller companies often lack the skills, financing or depth to make improvements needed to improve performance. A wider problem is that almost all the tests developed by the larger, more capable companies have been developed with the Western market in mind and are therefore unsuitable for wide use in TB-endemic countries due to their cost and high-tech nature. Nevertheless, promising new technologies do exist and, with adaptation, could be widely implemented in low-income settings. In an attempt to address this, the Tuberculosis Diagnostic Initiative (TBDI) was set up within WHO/TDR in 1996 to support industry and academic research into tests for resource-poor settings by providing an enabling infrastructure and a modest budget to fund development or evaluation projects. Despite offering substantial support to industry – including a 12,000 sample specimen bank for diagnostic testing; a database of all diagnostics in development; test performance guidelines; and support for field trials - this enabling approach was unable to drive the development process forcefully enough to deliver new tools and was supplemented in May 2003 by a new public-private-partnership, the Foundation for Innovative New Diagnostics (FIND). FIND is reviewing existing technologies, prioritising them from a health perspective and funding co-development of promising technologies with industry, in order to deliver new diagnostic tests for latent, active and MDR-TB. In conjunction with TDR, they will also trial existing (and eventually new) diagnostics to independently validate their performance. Diagnostic lessons This history of TB diagnostics research holds a number of lessons. The first is that it is not enough to support R&D indirectly: companies have little incentive to invest the estimated additional $5-20 million needed to conduct adaptation or development work and, in the 6.8-10 Chapter 6.8: Tuberculosis continued absence of such incentives, will not do so. Secondly, diagnostic development is increasingly the province of smaller companies and biotechs. Incentives targeted towards these companies will need to differ in size and scope from the incentives needed to stimulate multinational pharmaceutical companies. And, finally, the plethora of small companies, each promoting their own technology, can make it difficult for funders (or indeed purchasers) to determine where best to invest their funds. (For suggested solutions, see R&D Gaps, below.) Drugs A review of TB drug development needs to look at both industry and public/not-for-profit groups, since the balance of activity between these has changed dramatically. Industry All our current TB drugs were developed between 1940 and 1970, but there has since been a marked downturn in industry interest. Only three of the world’s Top 20 drug companies conduct any TB drug research or screen new compounds for anti-TB activity. And only one of these companies conducts TB research as part of its mainstream R&D activities, rather than as free-standing, smaller-scale research which tends to fall under the “corporate social responsibility” umbrella. d31 These three companies (all European) are GSK, Novartis and Astra-Zeneca. The chief disincentive to TB drug research by the multinational pharmaceutical industry is the lack of a sufficiently large commercial market for the final product. While global sales of TB drugs are potentially large (estimated at $450 million in 2000, expanding to $640 million in 2010), the key driver of commercial R&D investment is the private sector market in developed economies: for TB, this is estimated at only $113 million globally per year. 32 Although the major pharmaceutical industry has not developed any novel TB drugs in the past 30 years, they have produced two groups of drugs relevant to TB. The first consists of four drugs based on the rifamycins (discovered in the 1960s) and developed specifically to treat mycobacterial infections (M. tuberculosis and M. avium). All four of these were supported with US Orphan Drug funding although none are suitable for routine use in TBendemic settings for the reasons noted in Table 2.e Nevertheless, further development of one of these new drugs, rifapentin, may allow the frequency of drug administration, and therefore observation, to be reduced from daily to weekly or even fortnightly. In 2001, this was reported as five companies, however mergers have since reduced this to three companies. Orphan legislation has existed in the U.S. since 1982 and in the E.U. since 2000. Neither the US nor EU legislation mention neglected developing country diseases as a target, although the US later specifically noted these in the Pharmaceutical Exports Amendment Act (1986) to their Orphan Drug legislation. It is worth noting that, while neglected diseases are theoretically covered by the EU legislation, since even a few cases in the EC would qualify as a disease with prevalence less than 5 per 10,000, the original suggestion to explicitly include neglected developing country diseases in the legislation was blocked by EC Commissioner Bangemann. d e 6.8-11 Chapter 6.8: Tuberculosis Table 2: TB drugs developed by pharmaceutical industry 1980-200033 Drug Efficacy, safety, therapeutic benefit Rifater (Rifampicin, INH Pyrazinamide (120/ 50/ 300) Rifadin iv (Rifampicin iv) Combination of 3 old drugs (1952, 1965, 1970) Dosages do not comply with new WHO guidelines (150/75/400) WHO does not recommend use of 3-FDCs Intravenous form of old drug (1960’s) Oral treatment preferential, but useful in some circumstances Priftin (Rifapentin) Developed for AIDS opportunistic infection (M.avium complex) Used for once-weekly continuation phase TB treatment in some Western settings, where HIV can be excluded. Not suitable for HIV-TB co-infected patients in current dosage.. Prevention and treatment of AIDS opportunistic infection (M. avium complex) Useful for TB treatment in HIV patients on ARV therapy Very expensive Mycobutin (Rifabutin) US Orphan Drug support Yes 1994 Hoechst Marion Roussel (EU) Yes 1989 Hoechst Marion Roussel (EU) Yes 1998 Hoechst Marion Roussel (EU) Yes 1992 Adria Laboratories (US) A second promising group of drugs are broad-spectrum antibiotics developed by industry for Western infectious disease indications, but which incidentally have anti-TB activity. The fluoroquinolone family are particularly promising (e.g. moxifloxacin, gatifloxacin, ofloxacin), with public research from as early as the 1980s suggesting that the addition or substitution of fluoroquinolones in TB therapy could dramatically shorten treatment, from 6-8 months to around 3 months.34 However, until recently, their potential for TB treatment was not further explored nor were these patented drugs made available for large-scale public trials aimed at a TB indication. Companies were apparently deterred from working on a TB indication for these drugs (or assisting others to do so) because of the potential impact of a TB indication on their sales as broad-spectrum antibiotics in the West.35 However one major company, Bayer, recently moved forward on this and is now collaborating in publicly-driven TB trials of their existing broad-spectrum antibiotic (moxifloxacin). A further interesting area of industry activity is the foundation of specialised infectious disease research institutes which include TB in their remit: the Novartis Institute for Tropical Diseases in Singapore, launched in January 2003; GSK’s Tres Cantos research centre in Spain, launched in 2003; and Astra-Zeneca’s infectious disease research centre in Bangalore, India, launched June, 2003 (and which appears to have a more commercial focus). These institutes are currently focussed on the early pre-clinical stages of R&D. For instance, GSK and AstraZeneca each has three projects in the early discovery stage; and Novartis has announced that its Singapore Institute will cover pre-clinical stages only. Some of these groups have intimated that they may seek public involvement, possibly through PPPs, to deliver a finished anti-TB drug but, whichever is the case, their work will play a critical role in priming the drug pipeline. Given their small market share, smaller biotech companies currently play a relatively more active role in TB drug development (as opposed to drug discovery). For instance, a 2003 6.8-12 Chapter 6.8: Tuberculosis review showed that 4 of the 5 companies currently collaborating on, providing or developing TB lead compounds are biotechs: ActivBiotics, FasGen, Chiron and Sequella Inc., all USbased (See Table 3). Small contract research organisations (CROs) are also the main supplier of outsourced R&D services for the major drug development PPP, the TB Alliance. 36 Public and not-for-profit Private Sectors The bulk of TB drug development is now being done in the public sector and by the TB Alliance. Collectively, this R&D ranges from basic research through to the development of new TB drugs, including novel compounds and adapted existing antibiotics. (See Current Pipeline for full details.) Basic research Basic research led to the 1998 decoding of the TB genome by the Wellcome Sanger Institute; while the TB Structural Genomics Consortium (over 70 laboratories in 12 countries) (See annex 6.8.4) is now identifying and placing in the public domain, the genes involved in TB persistence, virulence and reactivation; and the 3-D structure of their corresponding TB proteins. This work has been a major breakthrough and is a first step towards identifying new TB drug and vaccine targets. Novel drugs Novel drugs offer the best hope for improved TB treatment, since they are likely to be potent against MDR-TB as well as drug-sensitive strains and latent TB. A significant contributor to novel TB drug development is the TB Alliance, the sole PPP working in this area. The TB Alliance, launched in 2001, has a portfolio of 10 anti-TB compounds or groups, including PA824, licenced from a US biotech and considered to be a promising TB compound because of its high bactericidal and sterilising activity.37 Two of the TB Alliance’s drug development projects are industry collaborations (rifalazil analogues with a US biotech firm, and pyrroles with an Indian generic company); while the remaining nine are collaborations with academic or public institutions (six of these are U.S. based). As of mid-2004, the TB Alliance had no major industry partners, although we believe discussions are ongoing.f Three smaller groups are also working on TB drugs (these projects include variations on existing drugs in distinction to novel compounds), however their efforts are focussed on development of a single drug or compound group held by an individual company, rather than being a broad-ranging portfolio approach aimed at identifying optimal drug leads. Two of these projects involve US biotech companies (Sequella Inc and FasGen, supported by the NIAID); while the third involves a major pharmaceutical company (development of thiolactomycin analogues by GSK, NIAID and St Judes). Adapted existing antibiotics The second main area of drug development involves trialling antibiotics already on the market, for a new TB indication. If successful, these drugs could be available relatively quickly, since they already exist as finished drugs; but, unlike novel TB drugs, they have the drawback of being potentially unusable against MDR-TB due to cross-resistance engendered Although not formal partners, GSH has seconded a senior scientist to the TB Alliance and BMS has donated $150,000 to the Alliance’s work. f 6.8-13 Chapter 6.8: Tuberculosis through previous widespread use for non-TB indications.38 The two most promising classes of drugs are the fluoroquinolones and longer-acting rifamycins (as noted above). If current large-scale clinical trials of these drugs substantiate early promising results, we may be able to reduce TB treatment in non-MDR settings to 3 months with 6-12 observations – as opposed to the current regimen of 6-8 months treatment with up to 80-100 observed doses. Despite evidence as early as the 1980s that these classes of antibiotics had potential for TB therapy, there was little progress due to both public inertia, based on the expectation that older TB drugs would suffice, and on the need for lengthy and sometimes unproductive negotiations with patent holders for access to drugs for clinical trials.g Since 2003, however, there has been an unprecedented increase in activity, with an October 2003 review showing 13 trials (including 5 Phase III trials) to assess the potential of fluroquinolones and longeracting rifamycins for first-line TB treatment. (See Table 3 Matrix.) The majority of these are being conducted by U.S. public health institutions, in particular the CDC and NIAID, although the EC and WHO/TDR are involved in at least one larger-scale trial. Developing country capacity to undertake these trials, including of novel drugs, is assisted by the preexistence of good DOTS delivery and recording systems. However, although trial capacity in TB-endemic settings is being rapidly built up (see p.13), it is still below the standard needed. Lack of funds has also been cited as one reason for delays in trialling « off the shelf » drugs, even though such trials are relatively cheap and the savings for developing countries are potentially substantial (trials of new drugs are estimated at $1.6-3 million for Phase II, and $8-22 million for phase III, depending on whether they are conducted in developed or developing countries.) g g 6.8-14 Chapter 6.8: Tuberculosis Table 3 TB DRUG DEVELOPMENT ACTIVITY MATRIX (Stop TB overview as of October 2003) Basic Research Discovery Lead Identification Pyridones and Quinolizines TB Alliance/KRIC T/Yonsei Lead Optimization Preclinical Clinical Trials Regulatory Inorganic Iron Compounds as TB Agents UFRGS Enoyl-ACPReductase (InhA) Inhibitors GSK(DDW)/TA M PA-647/PA-822 TB Alliance/NIAID PA-824-Preclinical Development NIAID(DAIDS)/JHU/T B Alliance and Others Moxifloxacin-Phase 2 Randomized MoxiContaining Regimen for Smear+TB (II) JHU/FURDJ/FDA Ofloxacin--OFLOTUB Comparative studies of OFL, MXF, GFL 4 mo vs. 6 mo St. George's/EC/TDR/WHO Shikimate Pathway Enzymes As Targets For Anti-TB Agents Development UFRGS Isocitrate Lyase (LCL) Inhibitors GSK(DDW) Ascididemin Compounds AUCKU/TB Alliance Thiolactomycin Analogs NIAID(TBRS)/GSK/St. Jude PA-824--Confirmatory Assessment of the AntiTB Activity of PA-824 in Mice JHU/TB Alliance/NJMRC Antimicrobials GSK(DDW) Third Generation Macrolides UILL/TB Alliance KRQ-10018 TB Alliance/KRICT/Yonsei Moxifloxacin--Shortening with MXF II JHU/CDC/NJMRC/Bay er Rifapentine (INH/RIF/EMB/PZA)- 4 mos vs. 6 mos in HIVnon-infected adults who are sputum neg. after first 2 mos TB treatment DMID 01-009 (III) DMID/NIAID/NIH (TBRU) Rifapentine-Randomized Weekly RIF/INH for 3 Months vs. 2x Weekly RIF/PZA for 2 Months (III) JHU/UCFF Target Identification and Validation NHLS/WITS/GSK/ UW/UBC/ Harvard/LSHTM 6.8-14 Chapter 6.8: Tuberculosis Basic Research Discovery Mechanisms of DNA Metabolism in Mycobacteria NHLS/WITS/NIAI D/RVC Development of in vitro test for sterilizing activity St. George's Studies on Drug Tolerance St. George's 40 Grants in Target Identification and Assay Development NIAID Lead Identification Lead Optimization Preclinical Clinical Trials Sigma70identifying transcription inhibitors ASTRA PA 20013 NIAID/JHU/FasGen, Inc. Moxifloxacin--Preventive Therapy of LTBI with MXF in the MDR Era JHU/CDC/NJMRC/Bay er Rifapentine-RPT/INH-3, INH/RIF-3, Continuous INH,Novel TB Prevention Regimens for HIV+ Adults (III) JHU/WITS/NIAID(DAI DS) Methyl Erythritol Pathway inhibitors (end product, isprenoids) ASTRA DNA Synthesis Inhibitors ASTRA Ethambutol AnalogsSecond Generation Ethambutol Antibiotics NIAID(DMID)/Sequella , Inc. Gatifloxacin- Highly Active Quinolones for Treatment of MDRTB (Gatifloxacin with Ethionamide) CNYRC/BMS Rifapentine (II) Dose Escalation Study CDC-TBTC MJH 98-I-81 & Analogs (Isoniazid Compounds) TB Alliance/Wellesley/CN YRC LL-3858 (Pyrroles) Lupin(TB Alliance) Rifapentine for Latent TB Infection CDC-TBTC Rifalazil Analogs ActivBiotics(TB Alliance) Rifapentine RIF/INH efficacy of once weekly (III) CDC-TBTC 6.8-15 Regulatory Chapter 6.8: Tuberculosis Basic Research The Regulation of Dormancy in M. Tuberculosis NHLS/WITS/UPEN N/NIAID/ PHRI/ROCK Discovery Lead Identification Lead Optimization Preclinical Clinical Trials Nitroimidazooxazines Analogs NIAID(TBRS)/TB Alliance Rifabutin-safety and efficacy of short course in HIV+ (II) CDC-TBTC Anti-Persister TB Drugs St. George's Capreomycin-Aerosol Capreomycin (I) TBRS/NIAID/Masan/Yo nsei EBA of Moxifloxacin, Gatifloxacin, Levofloxacin, Levofloxacin, Lindezolid (I) NIAID(DMID)/TBRU Isoniazid ResistanceIntermittent ShortCourse Therapy CDC-TBTC 6.8-16 Regulatory Chapter 6.8: Tuberculosis ACRONYMS EMB-ethambutol FQ-flouroquinolone rifapentine RMP-rifampicin AUCKU-Auckland ASTRA--AZIPL University AstraZeneca R&D India INH-isoniazid MXF-moxifloxacin BMS-BristolMyers Squibb BTRU-Brazilian Tuberculosis Research Unit DHHS-U.S. Department of Health and Human Services LSHT-London School of Hygiene and Tropical Medicine RTI-Research Triangle Institute CNYRC-Central New York Research Corporation CSU-Colorado State University CORU-Cornell University IUATLDInternational Union Against Tuberculosis and Lung Disease JHU-Johns Hopkins University KRICT-Korea Research Institute of Chemical Technology NIH-National Institutes of Health NJMRC-National Jewish Medical and Research Center SRI-Southern Research Institute PHRI-Public Health Research Institute UPENNUniversity Pennsylvania WellesleyWellesley College SKMOH-South Korean Ministry of Health UFRGS-Federal University of the Rio Grande Do Sul of TAM-Texas A&M University TB AllianceGlobal Alliance for TB Drug Development WITSUniversity of Witwatersrand OFL-ofloxacin PZA-pyrazinamide RIF- CWRU(TBRU)Case Western Reserve University Tuberculosis Research Unit DMID-Division of Microbiology and Infectious Diseases Masan-Masan National TB Hospital CDC-Centers Disease Control Prevention for and ROCKRockefeller University RVC-Royal Veterinary College St. George'sSt.George's Medical School TBRSTuberculosis Research Section Yonsei-Yonsei University Medical School TDR/WHO-Special Programme for Research and Training in Tropical Disease UBCUniversity British Columbia 6.8-17 FUR-TD-TBRU-Federal University of Rio de Janeiro-Thorax Institute-Tuberculosis Research Unit NHDP-National Hansen's Disease Program CDC(TBRU)Centers for Disease Control and Prevention Tuberculosis Research Unit FDA--U.S. Food and Drug Administration CDC-TBTCCenters for Disease Control and Prevention TB Trials Consortium GSK-GlaxoSmithKline NHLS-National Health Laboratory Service NIAID-National Institute of Allergy and Infectious Diseases St. Jude-St. Jude Children's Hospital of UILL-University of Illinois Chapter 6.8: Tuberculosis Drug development lessons The most striking lesson to be derived from this review of drug R&D is that the normal mechanisms to supply new pharmaceutical tools simply do not apply. In particular, the multinational pharmaceutical industry (with the notable exception of the three EU-based companies noted above) no longer plays a lead role in TB drug development. TB drug development by most multinational companies was minimal during the 1980s and 1990s and tended to focus on Western niche markets (e.g. drugs for HIV-TB co-infected patients on antiretroviral therapies); large companies tend not to develop potential TB compounds in their libraries; and they can be slow in providing access to existing antibiotics of potential TB interest. The few multinational pharmaceutical companies who do show interest (all European-based) are working in early pipeline areas – including the important discovery area - and some have indicated that they will seek public partnership to move compounds forward. Instead, R&D of novel TB drugs, including for MDR-TB, is increasingly the province of publicly-driven groups. For instance, a Stop TB review of known TB drug development (as opposed to discovery) projects underway at the end of 2003, showed that the majority (22) were being conducted without any industry collaboration. Likewise, just over half of the compounds being developed into new TB drugs derived from the public and academic sectors rather than from industry.h Commercial development of TB tools, where it exists, also tends to be within small biotech companies rather than large multinational pharmaceutical firms (MNPs), this holding true for both drug and diagnostic development. The predominance of biotechs reflects two factors that are not present for MNPs: biotechs do not systematically shy away from smallerscale markets, with several companies continuing to work on these “niche” diseases; and biotechs invariably need external funding partners, such as PPPs, to move their compounds along the pipeline. An unexpected but useful flow-on from this “new world” of TB drug development, is that many compounds that had previously languished on the shelves of biotech companies and academic research institutes – a significant waste of both public and private capital – are now being developed into useful new TB drugs and diagnostics. A less positive outcome is that the substantial capacity of most multinational pharmaceutical companies is longer turned towards TB drugs, which cannot compete with Western commercial sales. Vaccines The only TB vaccine, bacille Calmette-Guerin (BCG), was developed over 80 years ago. Its protective effect is limited and geographically variable, with studies showing a zero protective effect in India, ranging up to a 77% protective effect in the U.K.39 MNPs were active in only one of the fifteen novel TB drug development projects listed, compared to the biotech industry which was providing lead compounds or participation in four projects. h 6.8-18 Chapter 6.8: Tuberculosis Research into new TB vaccines has increased substantially since the early 1990’s – indeed, more than 170 TB vaccine candidates have been screened in animal models since 1997. These candidates fall into five main groups: Sub-unit vaccines (nearly half of all vaccine candidates), which include one or more mycobacterial components believed to induce protective immunity; Naked DNA vaccines, consisting of DNA encoding protective antigens plus various adjuvants; Live attenuated mycobacteria vaccines (including recombinant BCGs, attenuated strains of M.tuberculosis, and non-pathogenic mycobacteria such as M.smegmatis); Live attenuated non-mycobacterial vaccines, such as Salmonella or vaccinia virus; Improved BCG (prime booster) vaccines, which combine BCG with a novel candidate for improved efficacy. However, our incomplete scientific understanding of disease models of latency and persistence, or of the human immune response to M. tuberculosis, means that development of these new vaccines has been largely empirical, with current candidates representing virtually all known technological approaches to immunisation. The vast majority are also still in the laboratory modelling stage, with only three candidates in Phase I trials (20-50 human subjects). Large clinical trials of these vaccines also pose major problems given the current state of scientific and technical knowledge (see “Opportunities” section below). TB vaccine research is chiefly being conducted in the public sector and through PublicPrivate Partnerships (PPPs). The main players are: The Aeras Global TB Vaccine Foundation (formerly known as the Sequella Global Tuberculosis Foundation), a PPP founded in 1997 (US-based); The European Commission TB Vaccine Cluster (TBVac). TBVac is a consortium of academic and industry TB vaccine researchers, coordinated from the Institut Pasteur, and composed of 36 academic research groups from 12 EU countries, and two major pharmaceutical companies (GSK and Aventis). (See Annex 6.8.6 for full list); The Infectious Disease Research Institute, a PPP founded in 1993 (US-based); Several mid-size biotech companies who are developing TB vaccine candidates or technologies, often in conjunction with PPPs e.g. InterCell Corporation (Austria); and Corixa Corporation, Sequella Inc. and EpiVax Inc. (all US-based); Several major pharmaceutical companies, including GSK-Biologicals, Aventis and possibly Astra-Zeneca (all EU-based) who continue to work in the field of vaccines generally. The NIAID notes that major industry research is “relatively low level but steady”, although it can nevertheless play an important role.40 A multitude of academic researchers funded through public research grants. Vaccine lessons The upsurge in R&D into TB vaccines over the last decade has been made possible by recent advances in basic TB science, in particular sequencing of the TB and related genomes; development of new techniques for molecular manipulation of mycobacterial genomes; and progress in our general understanding of TB and its biochemistry, biology and disease mechanisms. These advances were, in turn, made possible through substantial increases in public and not-for-profit funding for TB research since the early 1990s. For instance, the US increased its TB research funding from $3.6 million in 1991 to around $60 million in 2001, 6.8-19 Chapter 6.8: Tuberculosis and the Wellcome Trust funded public discovery of the TB genome.41 Progress in the more general field of vaccinology, including by industry, has also been an important stimulus e.g. the use of DNA immunisation and adjuvant technologies. A second, and related, lesson is that our current state of scientific understanding and technical tools, while improved, is still insufficient to support rational, targeted TB vaccine development. The current plethora of empirical vaccine candidates stems not from a richness of proven opportunities, but rather from a lack of certainty as to where R&D efforts are best focussed. Finally, the difficulty and expense of vaccine development suggests future advances will continue to be through public-private collaborations, with no single group having sufficient resources to bring a TB vaccine to completion alone. Existing Resource Flows for TB R&D Basic research Most Western donors provide grants for TB basic research to academic and public health institutions. Total TB research funding (public and not-for-profit) is estimated by the WHO/TDR at approximately $125 million per year in 2000.42 As noted above, the US National Institutes of Health (NIH) are by far the largest investors, providing around half to two-thirds of funding in most years. The US Centers for Disease Control, and Wellcome Trust have also been major funders. The EC has been a more modest investor, providing Euro 28 million over 4 years for TB research under the Fifth Framework Programme (approximately $8.5 million per year at March 2004 conversion rates). An unspecified proportion of this goes to basic research (including structural and functional genomics, molecular epidemiology of MDR strains in Europe, and studies of the development of drug resistance).43 (See Annex 6.8.8) Diagnostics Development of new TB diagnostic tools was supported by a 2001 grant of $10 million over 5 years from the Bill and Melinda Gates Foundation to the TB Diagnostic Initiative within WHO/TDR. This was boosted in 2003 by a $30 million grant, also from the Gates Foundation, to set up FIND. The EC’s Fifth Framework programme provided Euro 13 million for drug and diagnostic R&D (the diagnostic proportion is not known), and the NIH also offers relatively small amounts (for example, approximately 0.6 million through Small Business Innovative Research Awards from 2002-2004). Expenditure by companies is unknown. Drugs Resources for TB drug development come from 3 sources: industry investment, PPPs and public investment. Although the three industry TB research institutes account for a relatively small proportion of the pharmaceutical industry’s current $40 billion annual spend on new drug research, they nevertheless represent a substantial contribution to global TB drug R&D. Astra-Zeneca invested $10 million to set up its 70-person Research Institute in India, with a $5 million R&D budget for 2001; Novartis and the Singapore Government are jointly funding the 70-person Novartis Singapore Institute with $25 million/year for 5 years (their respective contributions are unknown); and TB-dedicated resources at GSK’s Tres Cantos centre are unknown.44 6.8-20 Chapter 6.8: Tuberculosis Funding for Public-Private Partnerships is surprisingly small given their leading role in TB drug development. The only PPP for TB drug development - the TB Alliance- has raised $42 million since 2001, the bulk of this from the Rockefeller Foundation ($15 million pledged) and Gates Foundation ( $25 million grant). This sums are insufficient to finalise development of the TB Alliance’s most promising lead compound, let alone other compounds in its portfolio. Public investment in TB drug development takes a number of forms. Both EU governments and the US offer a wide range of general R&D incentives to the pharmaceutical industry including research subsidies, tax breaks and Orphan Drug market exclusivity. For instance, the UK instituted a 150% tax break for neglected disease research in 2002, and U.S. Orphan Drug incentives (commenced in 1982) supported development of the industry TB drugs noted above. The total costs of these financial incentives for industry are unknown, although thought to be substantial, nor do we know the proportion devoted to TB. The U.S. also offers in-kind support to industry (see the TAACF below). However, direct public investment in novel TB drug development is far more limited. The TB Alliance has received only one government grant (Euro 2 million from the Netherlands Gov’t) to develop its current portfolio of ten compounds; and the GSK-NIH-St. Jude’s project received a 3-year NIH Challenge Grant of $1.2 million with a matching contribution from GSK to develop thiolactomycin. Resources for the remaining two biotech projects are unknown, although the NIAID provides support to both. The EC Fifth Framework Programme provided Euro 13 million over 4 years for TB drug and diagnostic R&D, including for basic research and epidemiology, with an unknown proportion going to drug development. The US also provides in-direct support in the form of in-kind contributions to TB drug development. This is in the form of access to the NIH network of contract research organisations and grantees (the TB Alliance estimates this contribution at equivalent to $1.5 million); as well as access to NIH in-house research capacity, in particular the important services of the TB Antimicrobial Acquisition and Coordinating Facility (see boxed text). 6.8-21 Chapter 6.8: Tuberculosis The Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) The TAACF was established by the NIAID in 1994 to encourage and support academic researchers and pharmaceutical companies to re-enter the area of TB drug development. TAACF services are free to researchers anywhere in the world, including PPPs, industry and academic researchers; and data are kept confidential in order to protect the user’s intellectual property (IP). The overall service is managed by the Southern Research Institute (SRI), with individual services provided at five US-based centres, as below: High-throughput screening (HTS) of large compound libraries – in particular industry compound libraries - against validated TB targets. HTS has been provided by the SRI since 2001/2002. The SRI and collaborating institutions (e.g. Texas A&M University) also develop, validate and optimise target assays against which screening can be conducted. In vitro screening of promising individual compounds or compound groups, performed by the National Hansen’s Disease Program in Baton Rouge, Louisiana. This step is readily available to public researchers, who generally have the capacity to produce the small amounts of compound needed (usually between 1-7mg). In vitro screening is, however, slower and far more labour intensive than HTS, although this can be improved by robotisation of some steps. Compounds that pass all screens successfully are then referred for in vivo testing. TAACF data show that of more than 50,000 compounds screened, over 500 were successful « hits » and approximately 200 had sufficient in vitro potency and selectivity to warrant further testing as compounds with promising anti-TB activity. In vivo screening of compounds that have shown in vitro activity is performed by Colorado State University. Promising compounds are tested for their capacity to inhibit the growth of TB in an aerosol mouse model, using genetically modified mice that can deliver results in one month instead of the usual three. Medicinal chemistry and structural analysis services are also offered by the TAACF. These allow promising groups of screened compounds to be narrowed down to the most active compound structures within the group – the first step to identifying a drug lead. Technology transfer support is managed by the Research Triangle Institute (RTI). If the submitting group is interested, the RTI can help find partners and funding opportunities.to further develop promising anti-TB compounds identified by TAACF screening. 6.8-22 Chapter 6.8: Tuberculosis Discussion The TAACF is funded at around $2 million per year for all five facilities, and has now identified over 500 possible anti-TB compounds or « hits » from over 400 suppliers worldwide. This service has been invaluable, however the TAACF still faces challenges. To date, no large companies have used the TAACF’s High-Throughput Screening service (although it has also not been heavily marketed), apparently due to industry’s preference for keeping intellectual property in-house. In response, the TAACF have sought to purchase compound libraries, but note that this is very expensive, costing around $100,000 for a 100,000 compound library, plus an estimated additional $100,000 to put these through HTS. They are not currently budgeted to this level. An alternative approach suggested by TAACF staff might be to transfer TAACF-developed assays to industry, who could then conduct in-house screening. This approach would protect their proprietary knowledge – but industry would also have no obligation to divulge promising results (or negative results), or to conduct further drug development on any promising compounds discovered. In vitro screening has been popular, as witnessed by the figures above. The majority of users are academic groups (75%), with a further 10% of compounds coming from small companies such as biotechs. Only around 3% of compounds have been submitted by large drug companies, and often in response to a specific request by the TAACF (for instance, due to the TAACF following up an interesting literature report on a company-held compound). Industry are apparently reluctant to submit compounds since this would require them to reveal compound structures to the TAACF (the TAACF in turn requires structure disclosure as a precaution against patent infringement claims later in the day). A possible approach would be for the TAACF to conduct screens blind, however they would have no way of knowing which compounds were promising, or whether hits were being pursued. Many academic groups are unable to progress compounds past the in vitro stage, since they lack the capacity to scale-up production to the quantities needed for in vivo testing (up to 1gm of compound is needed), and are unable to produce the large numbers of analogues used for secondary screening of a promising compound or group. The TAACF has suggested that this could be overcome by providing government-subsidised access to Contract Research Organisations (CROs) who could provide these skills: these contracts could be extremely valuable to CROs. Finally, the TAACF’s medicinal chemistry services are warmly welcomed by non-industry groups but less relevant to industry which has superior experience in this area. The two most significant challenges identified by the TAACF were the inability to share information on promising « hits » with other researchers, since the TAACF is bound by confidentiality agreements; and the lack of any follow-on mechanism to ensure that promising hits are followed up by either academia or industry. Although some academic groups will publish their results, most have neither the incentives nor financial support to carry research forward into drug development. Likewise, our current system actively incentivises industry to keep information (either positive or negative) in house and, as noted above, does not highly reward investment in further TB drug development. Even if participants were willing to follow up all promising « hits », there is a further obstacle: no one is currently funded to do this work. While the TB Alliance certainly has the scientific expertise to identify the most promising « hits », its current under-resourcing means it lacks the capacity to include many additional early stage compounds in its portfolio. These latter obstacles are less easily overcome, since they hinge on the management of intellectual property. Solutions under consideration include a substantial incentive package for large pharmaceutical companies who have little to gain from a TB drug, but fear the loss of IP. Likewise, improved incentives to encourage academic researchers to relinquish or share IP would be valuable – and possibly less expensive. 6.8-23 Chapter 6.8: Tuberculosis Finally, governments provide indirect assistance through supporting clinical trials and building clinical trial capacity. This support is more evenly balanced between the EU and US, in particular since the launch of the European and Developing Countries Clinical Trials Partnership (EDCTP). Major groups now supporting TB trials include the CDC and NIH (e.g. through the NIAID, TBTC and TBRU); the International Union Against TB and Lung Disease; the EDCTP; and WHO/TDR, although the latter is constrained by resources. The EDCTP budget is EUR 600 million over 5 years for AIDS, TB and malaria; while the TDR’s budget is around $30 million/year for both drug development and research capacity building for all ten diseases in its portfolio.45 Vaccines Global investment in TB vaccine R&D was estimated at around $80 million per year in 2003, a figure which was dramatically increased by a recent large grant from the Bill and Melinda Gates Foundation (see below).46 The EC Fifth Framework programme allocated Euro 15 million (just under Euro 4 million per year) to vaccine research, chiefly through the EC TB Vaccine Cluster (TBVac). In addition, the EU is funding research into alternative vaccine delivery mechanisms (2 projects), and conducting early work to set up vaccine clinical trial sites in Africa (2 projects). The EDCTP also offers funding support at the Phase II and Phase III stages of clinical trials. The largest public supporter of TB vaccine research is, however, the U.S., in particular the NIAID, which provides: Academic grants for TB vaccine research (basic research and vaccine candidates) The TB Research Materials and Vaccine Testing contract, which provides: o high quality TB research reagents to investigators throughout the world. (One of the obstacles to TB vaccine research is the lack of quality reagents from the contagious and technically difficult TB mycobacterium.); o screening of TB vaccine candidates (over 170 candidates already screened); The TB Research Unit (TBRU) supports an international collaboration of scientists working on immunological markers of infection; surrogate markers for vaccine trials; and clinical trial capacity building; Infrastructure for Phase 1 and 2 human trials is made available to industry and public researchers (via the TBRU and the Vaccine and Treatment Evaluation Units; as well as the CDC’s TB Trials Consortium). Further U.S. support is provided through: The CDC (as above); The U.S. Food and Drug Administration, which will assist manufacturers in developing and licencing any new TB vaccines. Private not-for-profit groups are increasingly major funders of TB vaccine research, including: The Bill and Melinda Gates Foundation, who donated just under $83 million to the Aeras Global TB Vaccine Foundation in February 2004. Infectious Disease Research Institute (budget unknown) 6.8-24 Chapter 6.8: Tuberculosis Resource flow lessons This review of resource flows holds a number of lessons. The first is that government funding at the basic and clinical trial stages is relatively well-developed, and increasingly shared between the EU and U.S., but that funding for development of new TB tools – especially drugs and diagnostics - is, to be honest, a bit of a mess. Government financial incentives that do exist are largely aimed at and designed for the wrong target i.e. in-house R&D by multinational pharmaceutical companies (MNPs). However, the majority of MNPs no longer have scientific expertise in TB and have expressed a clear disinterest for working in this field; while those who are involved, have mostly shown a preference for working through PPPs or in public collaborations rather than developing drugs entirely in-house. Industry financial incentives are also poorly designed for the smaller biotechs, medical technology firms and Contract Research Organisations (CROs) who are now the main industry players in TB research: for instance, tax breaks are of little or no interest to companies with negative or small profits; and market exclusivity and patent extensions are of limited relevance to one-product companies. There is also a striking lack of public funding for the groups who driving development of novel TB tools, in particular the TB Alliance, which dominates the field. Indeed, current TB drug development – vital if we are to control TB and MDR-TB - is resting on the shaky foundation of two philanthropic donations and a handful of in-kind support from the U.S.. . A second major lesson is that the EU does not appear to have positioned itself strategically in terms of new TB tools, leaving the U.S. to play a dominant role. For instance, the EU provides less than 5% of the funding dedicated to TB drug development projects and, unlike the U.S., offers neither in-kind nor infrastructural support for most stages of drug development. No EU academic groups, public health institutes or biotech companies collaborate in TB Alliance projects, although a small number of EU institutions are members of the TB Alliance’s expert advisory groups (See Annex 6.8.7 for list). The bulk of funding for new TB tools comes from U.S. philanthropists; and drug development is dominated by U.S. research institutions (7 of 11 projects) and biotechs (all U.S. based). Trials to adapt existing antibiotics for TB are also dominated by U.S. institutions, which were conducting 12 of the 13 projects identified by Stop TB as of end 2003. (See Table 3 for full details.) What is the Current Pipeline? TB Diagnostics As noted above, there is a reasonably high level of activity in TB diagnostics, with new tests at every stage of the R&D pipeline from proof of principle through to Phase III trials. Although currently too expensive and technically difficult for resource-poor TB settings, many tests have potential for such use, if adapted. Diagnostics of particular interest include: a) Rapid culture and DST systems (rapid detection of organisms and drug resistance) Oxygen quenching MGIT (Becton Dickinson, USA) Colorimetric liquid culture (Biotest, Germany) Nitrase reduction assays (Sweden, Russia) Colorimetric solid culture (TK medium, Turkey) 6.8-25 Chapter 6.8: Tuberculosis b) Culture surrogates (for case detection and drug resistance) Phage amplification: FastPlaque (Biotec, UK) Luciferase reporter-phages (Sequella Inc, USA) c) Molecular methods (nucleic acid amplification for the detection of TB organaisms or for rapid detection of rifampin resistance) Inno-LIPA (Innogenetics, Belgium) PCR (Roche, Switzerland) SDA (Becton Dickinson, USA) BioChip (Englehardt, Russia) TMA (GenProbe, USA) d) Tests detecting antigen or volatile gases. This work is at an earlier stage, just short of proof of principle and, although technically challenging, is considered promising. Blood based tests to detect TB antigens (KIT, Netherlands; Univ. Bergen, Norway; Lionex Diagnostics GBH, Germany; Pasteur, Madagascar) “Electronic noses” to detect antigens in breath (KIT, Netherlands; Cranfield University, UK). e) Tests for latent TB infection, capable of distinguishing it from prior BCG vaccination or infection with non-tuberculous species of mycobacteria. Whole blood IFN ELISPOT (Oxford Immunotec, UK) f) Laboratory free tests for active infection using transient cellular immunity Skin patch test using MPT64 (Sequella Inc., US), now in Phase II trials TB Drugs As seen from the Matrix below, all stages of the TB drug pipeline are thin. The discovery and lead identification stages, which provide new compounds to enter the TB pipeline, have only a handful of projects; and the few compounds that are in development tend to be at the earliest pre-clinical stages. This means we cannot expect a novel TB drug, active against both MDR-TB and drug-sensitive TB, until at least 2010. From a first glance at this matrix, the late pipeline appears to be relatively full, however it should be noted that these products are existing antibiotics in clinical trials for a new TB indication, rather than genuinely novel anti-TB drugs. The final products will therefore probably be unsuitable for MDR-TB use, although they are promising in terms of simplifying and shortening non-MDR TB treatment, and may be available as early as 2007-2008. See Table 3 matrix. TB Vaccines Development of new TB vaccines has reached an important turning point, with a decade of experimental laboratory modelling now leading to entry of the first vaccine candidates into clinical trials. If successful, delivery of a final vaccine will take at least 10 years (2014). 6.8-26 Chapter 6.8: Tuberculosis Vaccine type Recombinant MVA vaccinia) Ag85A Developer (modified Live attenuated recombinant BCG rBCG30 Subunit vaccine/adjuvant (M72S) (used as either a BCG booster or an improved BCG) Subunit vaccine/adjuvant - ESAT-6 plus Ag85B Multi-epitope subunit vaccine/adjuvant Oxford University Wellcome Trust (UK) TBVac Aeras Global TB Vaccine Foundation UCLA, U.S. NIAID NIAID IDRI Corixa Corporation Licenced to GSK (Possible EDCTP and Aeras interest in trials) Staten Serum Institute (Denmark) InterCell Corporation Aeras Foundation Clinical trial stage Phase I, in UK and Gambia (commenced 2001) Phase I subjects) clinical trials (30 Phase I subjects) clinical trials (20 Possibly Phase I in 2005 Uncertain progress: probably be abandoned will Opportunities for Research into New Pharmaceutical Interventions a) What is the state-of-the-art science for TB – what opportunities does it offer? The greatest opportunity – and one which is realistic and achievable – is to apply our existing scientific and technological skills to develop families or groups of compounds or technologies already known to have anti-TB potential, but which have not progressed due to lack of political will and funding.47 (See Appendix 6.8.1) Seizing this opportunity alone would deliver a broad range of new tools to address the growing threat of TB in Europe and elsewhere. Three opportunities stand out: Completion of promising novel drugs already in the pipeline; Adaptation of existing TB diagnostics for use in resource-poor settings, including tests for screening, diagnosis and MDR-TB detection ; Further investigation of compounds with promising anti-TB activity, and screening of industry compound libraries to find remaining anti-TB compounds that have not been examined and taken forward. Scientific advances have also opened up a number of new opportunities, as listed below. The most significant breakthrough has been the decoding of the TB genome and other mycobacterial genomes, and follow-on work by the TB Structural Genomics Consortium to identify the TB genes and associated proteins responsible for latency, persistence and activation of TB (the first 40 proteins have already been elucidated).48 This work, once completed, will be the first step towards rational design of new TB tools, rather than the resource-intensive empirical approach currently used to develop new drugs and vaccines. The development of TB fixed-dose combinations (FDCs), rather than single-drug formulations, has also been a major therapeutic advance, in particular because of the role FDCs play in reducing maladministration and consequent MDR-TB. Key areas for further research include: 6.8-27 Chapter 6.8: Tuberculosis Basic science49 Improved understanding of how TB “works” in the human host, in particular disease models of latency, persistence and re-activation; Improved understanding of the human immune response to M. tuberculosis, including: the role of various T-cell populations; the molecular signals that activate the protective immune response; and the TB antigens that induce human protective immunity. Diagnostics Increased discovery research into new diagnostic approaches Increased research into new delivery methods, for example the “electronic nose” concept noted earlier. Drugs Research to sift through TB target proteins (target validation), including those now coming out of the TB Structural Genomics Consortium, in order to identify the most promising targets for drug and vaccine development.50 Development of surrogate markers that can act as early end-points for success or failure during clinical drug trials (we now have to follow patients for 2-years to rule out treatment failure). Use of surrogate markers would substantially reduce both the cost and length of clinical trials, expediting the arrival of new drugs.51 Aerosol drug delivery mechanisms that promise to rapidly debulk pulmonary TB and to extend our range of treatments to drugs that cannot be administered orally because of poor pharmacokinetics or side-effects of high system exposure. However, work in this area is early, and use may be limited by the expense of delivery devices.52 Depot formulations of existing TB drugs, which offer the promise of once-monthly administration instead of observed daily administration, delivering significant benefits in terms of adherence, reduced creation of MDR-TB and lower TB programme costs. Reports on depot preparations were published as early as 1966, with follow-up studies by the University of Illinois showing that a single subcutaneous polymer depot implant of INH and pyrazinamide delivered the same therapeutic activity as daily administration over 8 weeks.5354 Industrial technologies for depot preparations already exist (e.g. polymers or microspheres), as do the component drugs, making this an interesting area for investigation. Slow-release oral formulations of existing TB drugs, which maintain plasma levels for around 3 days, theoretically allowing twice-weekly dosage. Research in this area has been conducted since 2001 by the Postgraduate Institute of Medical Education and Research, Chandigarh, India. As with depot preparations, the technologies and component drugs already exist, again making this a promising area for further research. Development of further fixed-dose combinations, in particular combinations that include second-line drugs and new drugs that come out of the development pipeline. Vaccines55 Development of surrogate markers of vaccine-induced immunity. In their absence, clinical trials to establish immune status are likely to be very expensive, requiring “long duration and/or enormous cohort sizes”, for instance patient follow-up would need to be an estimated minimum 3-4 years post vaccination;56 6.8-28 Chapter 6.8: Tuberculosis Lack of cheap, simple diagnostics to distinguish BCG-vaccinated patients from those with latent or active TB. In their absence, “identification of the appropriate patient population remains a challenge” – a huge obstacle to clinical trials – with diagnostics being described as “essential tools for...clinical evaluation of candidate vaccines”;57 Improved vaccine adjuvants; Improved animal models to better mimic the action of TB in humans (eg. persistence, reactivation and the cross-effect of non-pathogenic and environmental bacteria, and of prior BCG vaccination); Identification of the optimal route for vaccination (nasal, oral, intradermal etc.)58 b) What is the current status of institutions and human resources available to address TB ? Structural genomics The TB Structural Genomics Consortium, set up in 2000. The TBSGC is composed of 70 member laboratories in 12 countries around the world, supported by central facilities at three U.S. sites. Just under a third of member institutes are located in EU Member states. (See Annex 6.8.5 for full list). Basic research Informal institutional support, funded through grants from national governments and institutions, with the US being the predominant funder; The TB Alliance provides some assistance (e.g. through establishment of expert working groups and seminars).59 TB diagnostics TDR (which plays a supportive role) FIND (PPP): active review and prioritisation of technologies and co-development of new TB diagnostics with industry Neither of these addresses discovery research, which is largely being supported (under-supported) through ad-hoc national research grants Limited NIH support Within the EU, there are 28 biotech companies, and at least 5 academic institutions and 4 public institutes, working on TB diagnostics. (See Annex 6.8.6 for list.) TB drugs The TB Alliance (PPP): active review of promising compounds and development of new TB drugs Broad NIH support, including through the TAACF A list of EU-based academic institutions and industry groups working in this area is attached at Annex 6.8.7 There is limited institutional support for research into alternative drug delivery mechanisms, which is largely conducted through ad-hoc national research grants, including: o Animal studies of aerosol rifampicin (academic groups in U.S., India and UK);60 and a later stage human trial of aerosol capreomycin for MDR-TB (NIAID/TBRS/Masan/ Yonsei: see Table 3); o We are not aware of any formal institutional support for research into depot or slow-release drug preparations, although industry (including small to midsize firms) is very active in the high-growth area of alternative delivery 6.8-29 Chapter 6.8: Tuberculosis technologies.i Two academic groups working in this area are the University of Illinois (US) and the Postgraduate Institute of Medical Education and Research, Chandigarh, India. Target identification and validation research: o Some institutional funding from NIAID (40 grants) See Table 3 Surrogate markers for drug and vaccine trials The NIAID provides some funding (e.g.TBRU-Case Western Reserve UniversityMakerere University Uganda project); GSK previously had a joint project with Stellenbosch University (South Africa) under its Action TB initiative; Informal support for academic research, funded through grants from national governments and institutions. Vaccines Aeras Global TB Vaccine Foundation (PPP) The Infectious Disease Research Institute (PPP) The European Commission TB Vaccine Cluster (see Annex 6.8.6 for member list); Broad NIH support (see above) Adjuvant development is moderately well supported, with significant industry activity (biotechs and major companies) and some public grants (e.g. NIAID); Alternative delivery models receive very little support, and mostly through ad-hoc research grants (e.g. the EC funds 2 projects; NIH grant to Uni. North Carolina) Are there research gaps that could be filled affordably, have a significant impact and be achieved in a) 5 years or b) the longer term? Which of these are pharmaceutical research gaps? Investment into TB research offers two main opportunities: areas where relatively small investments can deliver large returns; and areas where investment into a research gap or bottleneck will deliver increased flows throughout the R&D pipeline. These are outlined below. Basic research A shortlist of validated molecular targets would revolutionise TB drug development by allowing industry to move towards rational drug design in addition to current methods (e.g. methodical examination of compounds with known anti-TB activity). There have been dramatic scientific steps in this direction, with the TB Structural Genomics Consortium now examining the genetic basis of TB latency and persistence, and systematically publishing the structure of TB proteins (some of which will be valuable drug targets). However, we will A 2004 conference noted that drug delivery systems and emerging technologies accounted for $38 billion in revenues in 2002, with expected growth of 28% per annum over the next 5 years. Conference Ref : Drug Delivery Systems, Europe 2004 - Business Development, Emerging Technologies Novel and Niche Delivery Methods; 25th & 26th February 2004, London. Accessed March 2004 at http://www.visiongain.com/b2b/Drug_delivery.htm i 6.8-30 Chapter 6.8: Tuberculosis not be able to capitalise on these advances unless we increase our input in two areas: target identification and validation; and further research into disease models of latency and persistence.61 Target identification and validation is the process of sifting through TB proteins to identify those most likely to be useful for drug development (most relevant to the disease process and most technically tractable). This work is in turn facilitated by a better understanding of the principles of latency, persistence and reactivation, and of the human immune response to M.tuberculosis infection. The time is ripe for intervention in this area, since the TB Structural Genomics Consortium (TBSCG) is already delivering TB protein structures, which will need to be prioritised in terms of their relevance to TB disease mechanisms and many EU groups are already involved in the TBSCG. Diagnostics Diagnosis is currently the rate-limiting step in entry to TB treatment, with less than half of all patients with active infection being diagnosed and treated. Within an expanded EU, there is also an urgent need for cheap, rapid and reliable tests for TB screening (CXR is notoriously unreliable) and MDR-TB diagnosis (ideally within 3 days). The development and implementation of such tests is well within reach. There are promising TB diagnostics at all stages of the pipeline, and a plethora of lowhanging fruit in the form of existing tests that need relatively cheap and minimal adaptation before they could be used in TB and MDR-TB endemic settings. Diagnostic development is far less resource-intensive than drug development, with an average new test costing between $5-20 million to develop, compared to the estimated $800 million needed for fully industrial development of a new drug (adaptation of existing diagnostics is even cheaper). Development times are also far shorter, at around 1-3 years compared to 10-15 years or more for new drugs or vaccines. FIND expects to produce the first new field-relevant diagnostics in 2005-2008, with a stream of further trial results over the next 5 years. EU support for new TB diagnostics has additional merits beyond the obvious need, and the cost-effectiveness and feasibility of new tests. The EU already hosts a number of TB diagnostic firms (28), as well as a network of Supranational Reference Laboratories (SRLs) tasked with supervising MDR-TB diagnosis in laboratories in Eastern and Central Europe, FSU and developing countries.j Renewed TB diagnostic activity is now centred in Europe, with both FIND and the TDR located in Geneva; and the mechanics of any EU intervention would be significantly simplified by the presence of a single PPP in the diagnostic field (FIND), which has reliable start-up funding and well-established links with pharmaceutical firms working in diagnostics. FIND offers the added advantage of having the scientific expertise (including senior staff attracted from the CDC and TDR) to prioritise the confusing array of existing and new TB diagnostics, allowing R&D investments to be optimally targeted. (See Annex 6.8.6 for a list of EU-based firms with interesting technologies.) j SRLs exist in the UK, Sweden, Germany, Italy, Belgium, amongst others. 6.8-31 Chapter 6.8: Tuberculosis Finally, unlike R&D into TB drug development - which is already dominated by U.S. funding, researchers and industry - the field of TB diagnostics is still “up for grabs”. The NIH is currently looking at TB diagnostics to see whether it has a role to play, but its activity is still largely limited to blue skies research and field trials - in other words, it is an area where the EU has room to make its mark. Short-term, cost-effective results (< 5 years) could be achieved by: 1. Funding FIND directly, in conjunction with the Gates Foundation, to co-develop tests with industry. Such an approach would support EU industry, while keeping R&D firmly focussed on optimal public health outcomes; 2. Providing industry incentives to encourage and reward EU-based diagnostic firms who work with FIND to develop new field-adapted tests, including new tests for TB screening, and rapid (3-day) tests for MDR-TB. These incentives should be designed with biotech companies in mind, since these dominate the field; 3. Ensuring EDCTP support is allocated to trials of new and existing diagnostics, including through FIND and TDR. The EU could also fuel the longer-term diagnostic pipeline by funding the significantly under-supported area of diagnostic discovery research. This would in turn provide new leads, which EU-based diagnostic firms could develop for Western markets and adapt, in conjunction with FIND, for resource-poor countries. Possible approaches include: 4. Funding FIND to either a) extend its scientific pipeline to the discovery stage; or b) employing FIND as a technical advisory body to review existing approaches and provide the EU with information on which are most promising 5. Providing a formal funding stream for public research into TB diagnostic discovery and encouraging member states to do so. Conversely, the EU may wish to directly support the biotech industry by providing incentives for the industry to develop more sophisticated versions of any simple, new fieldadapted tests that appear, for instance by developing robotised assays for use in higherincome EU and Western markets. k Drugs The biggest gap in TB drug development is primarily structural rather than technical. While it is true that technical hurdles exist in identifying compounds, that the models of infection are challenging and that clinical trials take years to undertake, nevertheless given adequate A matter that is not related to R&D, but is nevertheless important to the EU. It would be extremely helpful if EU-based Supranational Reference Labs could be funded to conduct their supervisory role with respect to developing country TB laboratories, in particular laboratories in high MDR-TB areas of Eastern and Central Europe: this work is currently performed voluntarily. The lack of approved, high-quality national TB laboratories in some countries (e.g. Russia) makes it extremely difficult to either map or control MDR-TB in these settings and, by extension, the wider European forum. SRLs have repeatedly expressed willingness to increase their contribution in this area, if funded. k 6.8-32 Chapter 6.8: Tuberculosis human and financial resources these problems can be addressed. What is lacking is the commitment to overcome these problems. As noted above, some large pharmaceutical companies now play a significant role in TB drug discovery, while TB drug development is dominated by publicly-driven groups, in particular the TB Alliance, with the biotech sector playing a more minor role. The TB Alliance has shown itself willing and capable of developing new TB drugs, and the pharmaceutical industry (in particular biotechs and CROs) are equally willing to provide the necessary technical skills if paid or incentivised to do so. However, the EU currently has no financial tools to link these two groups together – neither direct funding for the TB Alliance, to facilitate outsourcing of contracts; nor subsidised Alliance access to industry in-kind services; nor incentives to encourage industry to offer in-kind services to the Alliance. Likewise, there is limited support for biotechs working in this sector, with many of current industry incentives being poorly suited to smaller companies. This lack of support for TB drug development, or for a role for EU industry in this development, is particularly surprising given the EU’s urgent need for more effective and affordable drugs to manage TB and MDR-TB in an expanded EU. The EU accession countries now report nearly 1,000 new cases of MDR-TB each year, and 4,721 cases of TB that are resistant to at least one TB drug.62 Differing costs between countries make the cost of treating these cases difficult to estimate, however, at Latvian prices, treatment costs for all new MDR-TB patients in the accession countries would be EUR 105 million in the next decade, assuming no increase in patient numbers or in annual treatment costs.63 We could probably double or even triple this figure since, as noted above, TB numbers are trending upwards at around 5-7% per year in the relevant countries; and costs will be far higher for patients who enter Western European health systems (the US estimates $250,000 per patient, compared to Latvia’s $12,500 per patient). In addition to MDR costs there are, of course, also the costs of providing lengthy DOTS treatment to the more than 50,000 cases of “normal” TB reported in Europe and the accession countries each year (over 80,000 cases if the second-line accession countries are included).l In the UK, “normal” TB treatment is estimated at $9,029 per patient, of which only $200 is drug costs.64 By contrast, the cost of developing a new drug that allows short, effective treatment of MDRTB (and “normal” TB) is estimated at $76-115 million from lead compound up to registration, including the cost of all failed projects.32 The final drug would be available not only for MDR-TB patients in Europe but for all TB patients worldwide.65 EU support for new TB drugs would not only cost-effectively address Europe’s substantial and growing TB problem but, if based on a well-thought out incentive or investment structure, could also support the competitiveness of the EU-based pharmaceutical industry (in particular biotechs and CROs) and their profitable involvement in TB drug development activities that are currently dominated by US companies and institutions. l By “normal”, we mean TB uncomplicated by HIV/AIDS or multi-drug resistance. 6.8-33 Chapter 6.8: Tuberculosis There are three main areas where EU investment in TB drug R&D represents “value for money”. These are: 1. Discovery of promising TB lead compounds 2. Lead optimisation and pre-clinical 3. Clinical trials of existing drugs. 1. Drug discovery research and lead identification. These first steps in the drug development process involve identifying the best approaches to a known target (eg. inhibitors of DNA synthesis), and narrowing these down to a group of active compounds (e.g. pyridones). These compounds can be discovered either by high throughput screening of large industry compound libraries, or by targeted in vitro and in vivo screening of compound families with known anti-TB activity. Medicinal chemistry techniques are then applied to screening results in order to determine which, among the many structures in a TBactive compound group, is most likely to be safe, effective and feasible i.e. a lead compound. These R&D areas are crucial, since relatively high numbers of lead compounds must enter the pipeline in order to deliver a single successful TB drug – yet, as of end 2003, there were only 6 discovery projects and 3 lead identification projects underway. (See Table 3) There is little direct public funding for discovery research, which is currently being conducted almost entirely by EU-based industry (GSK, Astra-Zeneca and Novartis) and falls outside the remit of the TB Alliance. The TB Alliance is more involved at the lead identification stage, and offers some support – for instance, it is developing a publicly-available database of the many known compounds with anti-TB activity; while the NIH also provides a free screening and medicinal chemistry service through the TAACF, which could theoretically screen these compounds (see boxed text). However, the TAACF’s services are ad-hoc rather than systematic, being provided at the request of academic institutions, biotechs or industry who are interested in and funded to do TB research. There is no systematic method to review all known promising compounds, nor is there systematic access to industry compound libraries, most of which remain closed to public researchers. Possible EU approaches could include: Setting up a consortium of European academic institutions and industry groups (perhaps similar to the successful TB Structural Genomics Consortium) who would systematically prioritise, and submit for screening, those compounds with known or suspected anti-TB activity, using the TB Alliance database as a guide; Supporting this consortium by providing a free EU-based facility for in vitro and in vivo screening, taking into account the TAACF experience. (This approach is not predicated on access to company-held intellectual property); Sub-contracting CRO’s to produce analogues of promising compounds (“hits”) identified by screening, with these hundreds of analogues then entering secondary in vitro screening; Sub-contracting CROs to conduct scale-up synthesis of promising “hits” to produce sufficient quantities for the next stage of testing (in vivo testing in mice); Providing incentives to encourage researchers to relinquish or share IP with groups who can conduct further drug development; 6.8-34 Chapter 6.8: Tuberculosis Supporting development of the TB Alliance database of TB-active compounds, to guide the work of the proposed consortium; Providing a public medicinal chemistry service to optimise screening results, similar to that provided by the TAACF. Or, given industry’s superior experience in this area, a better alternative might be to sub-contract this work to a loose network of EU-based CROs, biotechs and industry groups who are willing to participate; or to offer financial incentives to encourage firms to supply these in-kind services to not-forprofit drug development groups; Provision of a high throughput screening facility is less promising, given the TAACF’s experience to date. However, the EU could fund purchase of compound libraries by EU-based groups, for subsequent screening by the TAACF: this approach would allow costs to be shared 50:50 between the EU and U.S. and would facilitate involvement of EU companies and academics in TB drug development. There are several persuasive reasons for EU involvement in these areas. The structured approach suggested above would allow the EU to put in place a more productive model than the less structured, individual enterprise approach being followed by the U.S.. At the same time, it would provide financial and infrastructure support to EU-based academic and biotech institutions involved in the screening consortium; and would provide a range of lucrative opportunities to European CROs, biotechs and industry by purchasing their private sector skills for public use. Incentives or support at the discovery stage would also benefit EU-based multinationals who have a monopoly in this area. Finally, the paucity of discovery and lead identification research is now choking off R&D further down the drug pipeline: this alone is a major incentive for increased EU activity in this area. Discovery research and lead identification of new anti-TB compounds require larger and longer-term investments than pre-clinical research, with estimated costs, including costs of failure, ranging from $40 million (using a PPP model) to $125 million in a pure industry model. m 66 However, the EU would not have to bear the total cost since EU industry already makes substantial investments through their infectious disease institutes; and the EU could select the most cost-effective among the suggested options - for example, a targeted screening facility plus outsourced contracts for medicinal chemistry and scale-up production of “hit” compounds. These initiatives would, in turn, provide substantial benefits to EU academic networks and industry. 2. The lead optimisation and pre-clinical stages of drug development offer a prime opportunity for the EU to achieve these goals. Lead optimisation is the process of improving a promising anti-TB compound to make it more potent, more selective and more “drug-like”. The pre-clinical stage takes this optimised compound and conducts the toxicological and ADME studies (pharmacokinetic and absorption, distribution, metabolism and elimination) needed before clinical trialling in humans. These technologies are well established and readily available within industry, including contract research organisations (CROs). The pre-clinical stage of drug development is also cost-competitive, being estimated at $4.9-5.3 million for a successful drug, including costs of failure (these costs are based on a survey of CROs who provide these specialist services).67 Results are deliverable within 5 years. The wide range is explained by the difficulty of estimating discovery costs, since this information is generally kept in-house by industry. m 6.8-35 Chapter 6.8: Tuberculosis Possible mechanisms to facilitate industry involvement at these stages of TB drug development include: Direct funding of the TB Alliance, who would in turn outsource relevant activities to industry. Again, this supports industry activity, while allowing it to be closely targeted on tools of maximal public health utility; Industry incentives designed to maximally reward contributions to gaps in the drug development pipeline (ideally identified by the independent scientific experts e.g. the TB Alliance’s scientific experts committee ) rather than the current scattergun approach of broad-brush R&D subsidies; Industry incentives targeted to the needs of smaller European biotechs, CRO’s and medical technology firms, as well as those more suited to multinational companies; PPP access to industry groups who are already sub-contracted to EU public health institutions (if these exist). Any or all of the above measures would be a win-win outcome, supporting EU industry while using the scientific expertise of the TB Alliance or other public groups to ensure that subsidised industry activity was tightly targeted on public health outcomes of priority interest for the EU. 3. Clinical trials of existing industry antibiotics for a TB indication (thirteen such trials (five Phase III) have already commenced). Such trials are an extremely cost-effective use of public funds, since the cost of adapting existing drugs to a new indication is far lower than developing new drugs from scratch. They also allow private innovation by EU companies (Bayer, Aventis) to be extended to a broader public. Beyond supporting these trials financially, the EU could support the further industrial work needed to provide these drugs in fixed-dose combinations with other TB drugs (FDCs) rather than single tablets. The cost of Phase I to III clinical trials of a novel TB drug are estimated at $26.6 million in a developed country setting and $9.9 million in a developing country setting, and would take an estimated 7-10 years to complete (in the absence of surrogate markers).68 The length and cost of trials using existing antibiotics would be substantially lower since many of these trials are already well-advanced (in Phase II or Phase III) and partially funded, including by the EU. Two further areas with investment potential are a) non-pharmaceutical gaps in the pipeline and b) exploratory technologies noted above. a) Non-pharmaceutical gaps in the current pipeline e.g. developing country clinical trial capacity and regulatory issues. Clinical trial capacity building is not a clear area of comparative advantage for the EU. Nevertheless, it represents a “niche” opportunity due to the presence of a major new EU initiative (the EDCTP); the relevance of the final outcome to EU industry (e.g. the potential benefit of cheaper developing country trial sites); and the public health interests of EU members. Support should therefore continue, albeit with more resources applied more quickly. 6.8-36 Chapter 6.8: Tuberculosis Several gaps in the regulatory process for new TB drugs could also be usefully addressed by changes in EU policy, including joint negotiation of a global standard for regulatory approval of new TB drugs (with the WHO, FDA and others); institution of a formal fast-track regulatory package for new TB drugs (similar to that provided for many orphan drugs); and regulatory approval of surrogate markers (when available) as valid end-points to support drug registration. Policy work on the first two issues will need to commence almost immediately, in readiness for the first adapted TB drugs (some are already in Phase III clinical trials, meaning possible registration as early as 2007-2008). b) Exploratory technologies As discussed above, three areas of under-funded TB research are: work on surrogate markers, to allow TB drug and vaccine trials to be completed more cheaply and quickly; research into depot drug preparations, to allow reliable once-monthly administration; research into new aerosol delivery mechanisms for TB drugs. Of these, the first two are most relevant to the EU. The EU has a comparative advantage in surrogate marker research, since studies have been conducted by EU-based groups, including GSK and St George’s Hospital Medical School (UK); and this work could be brought to completion within a 5-year timeframe. Surrogate markers are also of great interest to industry(and public researchers because of their ability to dramatically cut clinical trial times and hence costs; as well as expediting the arrival of novel drugs for TB and MDRTB. We are not aware of any work on depot or slow-release oral TB drugs being conducted in Europe (there is also very little elsewhere), however the clear advantages of this method of administration in terms of reducing MDR-TB creation and cutting TB programme costs (both financial and human) nevertheless mark it as a priority. If their early promise is substantiated, depot or slow-release preparations could be in the field more quickly than novel drugs, since the component drugs already exist and there is already a substantial body of industry experience in this area. Vaccines A TB vaccine is the holy grail of cheap, effective TB control. However, the relatively early nature of TB vaccine research and the lack of clear front-runners in this expensive and lengthy process make investment in TB vaccines a difficult area for public funders. A useful approach could be to target government support onto research that has a broad application across TB tools and/or to selectively support specific platform technologies. Five key areas stand out: Basic research into the human immune response to TB infection, and into disease models of latency, persistence and re-activation, since these underpin both drug and vaccine development (see above); Improved TB diagnostics, without which clinical trials of new TB drugs and vaccines will be extremely difficult and expensive (see above); 6.8-37 Chapter 6.8: Tuberculosis R&D of surrogate markers of cure and protective immunity, vital if we are to reduce the cost and length of clinical trials (see above); Adjuvant development is a prime candidate for EU support, since much of this work is conducted within the industry sector (major and biotech) and the resulting technologies can be applied by industry to other vaccines, including those for Western markets. A useful EC intervention would be to incentivise and financially support industry adjuvant research (methods of ensuring this research is applicable to TB are discussed in the Drugs section above). Incentives could be rapidly put in place, and results could be expected in less than 5 years. Alternative vaccine delivery systems. Again, this is an area where EU academic institutions are already active; where under-funding is a problem; and where the impact of oral or nasal vaccines, over injectables, is substantial. In the mid-to-long term, funding will also be needed to manufacture vaccine lots for largerscale clinical trials. Manufacture will almost certainly need to be done by, or in collaboration with industry, which has the technical know-how to scale up production and to handle high volumes of potentially infectious biological material. At this stage, suitable incentives will be needed to encourage and reward industry participation in this commercially less competitive area. Conclusion The expanded European Union now has a substantial and increasing TB burden of more than 50,000 cases per year, around 10% of whom have TB which is already resistant to one or more of our existing drugs. Globally, TB control is also threatened by the upsurge in HIV-TB co-infected patients, who are straining current TB tools and approaches to the limit. Although new tools and approaches are being developed in all areas – including basic research, drugs, diagnostics and vaccines - progress is being delayed by lack of targeted funding and support, in particular from the EU which now provides less than 5% of global funding for new TB tools. The U.S. is driving the TB R&D agenda, particularly for new drugs, with the preponderance of research and development now being funded by the U.S. government and U.S. philanthropists, and with U.S.-based industry and academic groups being the main collaborators (and beneficiaries of R&D contracts). The EU could redress this situation by selecting optimal areas for investment, based on current funding gaps, the potential impact of any intervention, the likelihood of rapid results, and the opportunity for the chosen approach to support EU industry and academic institutions. Based on these criteria, the following areas are considered optimal: 1. Adaptation of existing tools : Diagnostics (little support, including from US) Clinical trials of existing antibiotics for a TB indication. Depot and slow-release preparations of existing TB drugs (almost no support) 2. Application of well-established technologies to further investigate and develop known or suspected anti-TB compounds including: 6.8-38 Chapter 6.8: Tuberculosis Establishing an EU consortium to prioritise and co-ordinate screening of known compounds in order to discover new TB drug leads Outsourcing relevant development work to industry (through public funding of PPPs to cover outsourced work; or via industry incentives targetted to gaps identified by public R&D groups or PPPs). Outsourcing areas could include preclinical and lead optimisation work, medicinal chemistry, scale-up production of screening compounds, analogue development etc, as discussed above Providing a public facility for compound screening, and possibly medicinal chemistry, available at no cost to industry and academic groups. 3. Support for technology research, particularly in areas where industry is the main player or has a substantial interest: Surrogate markers of treatment (for drug trials) and protective immunity (vaccine trials) Adjuvant technologies for vaccines. 4. Basic and discovery research that will feed into multiple R&D areas Latency, persistence and reactivation of TB in the human host The human immune response to M.tuberculosis Discovery research into new diagnostic and drug approaches. In addition, the EU should continue its support for the work of the TB Structural Genomics Consortium (which has provided breakthroughs in all R&D areas) and for clinical trial capacity-building in developing countries. An EU decision to support research in these areas would deliver badly-needed new tools to manage tuberculosis within its own borders and elsewhere. Importantly, it would also establish a distinctive European model for R&D funding. Features of this model would be: A systematic, rather than ad-hoc, approach to promising R&D candidates; Co-ordinated academic research in key areas; Constructive use of PPPs as a conduit to identify gaps in the development pipeline, allowing R&D funding to be targeted to industry or academic groups who are active in gap areas, rather than lower-priority activities; Capacity to link support and rewards for industry, including biotechs and Contract Research Organisations, to activities that most closely match public health goals. 6.8-39 Chapter 6.8: Tuberculosis References69 1 What is DOTS? WHO 1999; WHO/CDS/CPC/TB/99.270 Global Alliance for TB Drug Development website. Accessed Feb 2004 at http://www.tballiance.org/2_0_TheNeedforNewDrugs.asp 2 3 Global TB Control: Surveillance, Planning, Financing; WHO; WHO/CDS/TB/2003.316 ; p.10 & p.19 Anti-TB Drug Resistance in the World, Report No. 2, Prevalence and Trends; 2000; WHO, Geneva (WHO/CDS/TB/2000.278) 4 The World Health Report 2003: Shaping the Future; WHO; Annex Table 3, p.160. Accessed online 21 Jan 2004 at http://www.who.int/whr/2003/en/Annex3-en.pdf. 5 Dye C., Bleed D. & Hosseini M., Progress Towards Targets for Global TB Control, Presentation at the Hague, Oct 2003. Accessed Feb 2004 at http://www.who.int/gtb/policyrd/Dots_expansion/4dewg_hague_oct03/presentations/7_oct/dye.ppt 6 Surveillance of TB in Europe, Euro TB: Report on TB cases notified in 2001; KNCV, Institut de veille sanitaire, Saint-Maurice; Dec 2003; Fig. 5, p.57. Accessed Feb ’04 at http://www.eurotb.org/rapports/2001/text_tables_2001.pdf 7 Both figures from Dye C. , Progress in global TB control, with special reference to Europe; Presentation November 2003, Slides 9 & 10. Accessed February 2004 at http://www.who.int/gtb/meetings/euro_workshop_nov03/en/presentations/introduction.ppt 8 TB deaths increasing in Eastern Europe; Press Release WHO/48; 20 06 94; “’Merlin’ will cure tuberculosis within two months? That is a fantasy”; Article from Konsomolskaya Pravda, Anatoliy Maksimov, January 15, 2004 9 Task force on communicable disease control in the Baltic Sea Region, Council of the Baltic Sea States. Accessed Feb 2004 at http://www.baltichealth.org/balticproject/project.php?id=43 10 Anti-TB Drug Resistance in the World, Report No. 2, Prevalence and Trends; 2000; WHO, Geneva (WHO/CDS/TB/2000.278) 11 Open Society Institute, International Harm Reduction Development Programme. Accessed Feb 2004 at http://www.soros.org/initiatives/ihrd/news/real_life 12 13 WHO Report 2003: Global TB Control; WHO (WHO/CDS/TB/2003.316). Respective country annexes. 14 Global Plan to Stop TB. Stop TB Partnership (2001); WHO/CDS/STB/2001.16, p.53. 15 Global Plan to Stop TB; op.cit; p.38 WHO website on DOTS-Plus and the Green Light Committee. Accessed at http://www.who.int/gtb/policyrd/DOTSplus.htm on 8 March 2004. 16 17 Global Plan to Stop ; op.cit., p.22 Progress towards targets for global TB control; Conference presentation by Chris Dye, Stop TB Partnership, WHO; Slide 18. Accessed Feb 2004 at http://www.who.int/gtb/policyrd/Dots_expansion/4dewg_hague_oct03/presentations/7_oct/dye.ppt 18 19 Global Plan to Stop TB; op.cit; p.62; Dr Gijs Elzinga, chair of the Stop TB Global TB/HIV Working Group; Presentation at Global Alliance Stakeholders’ Meeting, Paris, 30 Oct 2003 20 6.8-40 Chapter 6.8: Tuberculosis Dye C, Williams B et al, Erasing the World's Slow Stain: Strategies to Beat Multidrug-Resistant Tuberculosis. Science, 15 Mar 2002, Vol 295: p.2042; Global Alliance for TB Drug Development website, accessed Feb 2004 at www.tballiance.org/2_1_2_MDR_TB.asp 21 O’Brien R & Nunn P, The need for new drugs against tuberculosis, Amer J of Resp and Crit Care Med, Vol 163, No.5, Apr 2001, p.1056 22 Guidelines for Susceptibility Testing of Second-line Anti-TB drugs for DOTS-Plus, WHO/CDS/TB/2001.288, Geneva, p.1 23 Mohapatra P, Janmega A et al ; Second-line treatment for chronic TB ; The Lancet ; Vol 360, 9343 : 2 Nov 2002 Correspondence (40% cure rate); Task force on communicable disease control in the Baltic Sea Region, Council of the Baltic Sea States(67% cure rate). Accessed Feb 2004 at http://www.baltichealth.org/balticproject/project.php?id=43 24 O’Brien R & Nunn P, The need for new drugs against tuberculosis, Amer J of Resp and Crit Care Med, Vol 163, No.5, Apr 2001, p.1056 25 Abstract from full table, Executive Summary for the Economics of TB Drug Development, Global Alliance for TB Drug Development, Oct 2001, p.8. 26 Task force on communicable disease control in the Baltic Sea Region, Council of the Baltic Sea States. Accessed Feb 2004 at http://www.baltichealth.org/balticproject/project.php?id=43 27 Dye C. et al; What is the limit to case detection under the DOTS strategy for TB control?, Tuberculosis, Vol 83, Issues 1-3, 2003, pp.35-43 28 29 WHO Global TB Control Report 2003, p.30-32 30 WHO Global TB Control Report 2003, p.32-33 Fatal Imbalance, DND Working Group and Campaign for Access to Essential Medicines, Geneva, Sep 2001, p.12 31 32 Global Alliance for TB Drug Development, Economics of TB Drug Development; Oct 2001, p.10 US Food and Drug Administration, List of Orphan Designations and Approvals. Accessed Dec 2003 at http://www.fda.gov/orphan/designat/allap.rtf 33 Tsukamura et al; Therapeutic effect of a new antibacterial substance ofloxacin (DL8280) on pulmonary tuberculosis. Am Rev Respir Dis 1985; 131(3): 352-6 34 35 Barry, CE, Current Opinion in Investigational Drugs 2001, 198-201 36 GATB internal report October 2003 (unpublished) NIAID (http://www.niaid.nih.gov/newsroom/releases/PA824.htm) and the GATB Annual Report 2002-2003, p.9 (http://www.tballiance.org/pdf/TBA_annual_2002-2003.pdf ) 37 Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis and resistance; Lancet Infect Dis 2003 Jul;3(7) :432-42 38 NIAID Division of Microbiology and Infectious Diseases; TB Vaccines: State of the Science. Accessed Feb 2004 at http://www.niaid.nih.gov/dmid/tuberculosis/plan.htm; Colditz GA, Brewer TF et al. Efficacy of BCG vaccine in the prevention of tuberculosis: Meta-analysis of the published literature; JAMA. 1994 Mar 2;271(9):698-702 39 NIAID Division of Microbiology and Infectious Diseases; TB Vaccines: State of the Science. Accessed Feb 2004 at http://www.niaid.nih.gov/dmid/tuberculosis/tbvaccine.htm 40 NIAID, The Jordan Report 20th Anniversary:Accelerated Development of Vaccines 2002; p.173. Accessed Mar 2004 at http://www.niaid.nih.gov/dmid/vaccines/jordan20/default.htm 41 6.8-41 Chapter 6.8: Tuberculosis WHO : Strategic Direction for Research; http://www.who.int/tdr/diseases/tb/direction.htm. Accessed 3 March 2004 42 EC European Research Area Fact Sheet: EU-funded research in the fight against Tuberculosis. Accessed 3 March 2004 at http://europa.eu.int/comm/research/press/2003/pr2403en.html 43 AstraZeneca press release ; 1 March 2004. Accessed Mar 2004 at http://203.195.197.134/azeneca/newsinvest.htm 44 European Commission Decision 1209/2003/EC; 16 June 2003 ; Accessed Feb 2004 at http://europa.eu.int/eur-lex/pri/en/oj/dat/2003/l_169/l_16920030708en00010005.pdf ; TDR figures from Fatal Imbalance, op.cit, p.21 45 TB vaccine research doubled.The Scientist, Feb 19 2004. Accessed Mar 2004 at www.biomedcentral.com/news/20040219/03 46 WHO/TDR The current anti-TB drug research and development pipeline. Alan Hudson, Toshiko Imamura, Win Gutteridge, TOm Kanyok, Paul Nunn. TDR/PRD/TB/03.1W Geneva 2003 47 Mycobacterium tuberculosis Structural Genomics Consortium website. Accessed 8 March 2004 at http://www.doe-mbi.ucla.edu/TB/index.php 48 Ginsberg A, What’s new in tuberculosis vaccines? Bulletin of the WHO 2002, 80(6) :486; NIAID, The Jordan Report, op.cit; p.175; GATB internal report October 2003 (unpublished) 49 50 Duncan K, Progress in TB drug development and what is still needed; Tuberculosis (2003) 83:205 51 GATB internal report, op.cit; Duncan K (GSK)., op.cit., p.205; Ginsberg A, op.cit.; p.486 52 Duncan K., op.cit., p.203 Rizzo A, Moura N, Magalhaes M et al; Depot drugs in the treatment of resistant forms of pulmonary tuberculosis. The experience of the Conjunto Sanatorial Oct. de Freitas (Recife) ; Rev Bras Med. 1966 Dec;23(12):850-2 53 Gangadharam PR, Int J Tuberc Lung Dis 1999; 3(6):515-20. Gangadharam PR, Lailasam S et al; Experimental chemotherapy of TB using single dose treatment with isoniazid in biodegradable polymers. J Antimicrob Chemother. 1994 Feb;33(2):265-71 54 Research areas were identified by the following (all groups noted similar issues): WHO Initiative for Vaccine Research (IVR), State of the art of new vaccines: research & development. Accessed March 2004 at http://www.who.int/vaccine_research/documents/new_vaccines/en/index3.html; The Jordan Report, op.cit., p.176; Ginsberg A, op.cit, p.486; NIAID Division of Microbiology and Infectious Diseases; TB Vaccines: State of the Science, op.cit, pp.4-5 55 WHO Initiative for Vaccine Research (IVR), State of the art of new vaccines: research & development. Accessed March 2004 at http://www.who.int/vaccine_research/documents/new_vaccines/en/index3.html 56 57 The Jordan Report, op.cit; p.175-176 EU-funded research in the fight against Tuberculosis; EU press release. Accessed March 2004 at http://europa.eu.int/comm/research/press/2003/pr2403en.html 58 59 GATB Oct 2003 internal paper, op.cit Vyas SP, Kannan ME et al ; Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages ; Int J Pharm. 2004 Jan 9 ;269(1) :37-49 ; Tsapis N, Bennett D. et al ; Dierct lung delivery of PAS by aerosol particles ; Tuberculosis (Edinb). 2003 ;83(6) :379-85 ; Suarez S, O’Hara P et al ; Respirable PLGA microspheres containing rifampicin for the treatment of TB ; Pharm Res. 2001 Sep ; 18(9) :1315-9 and many others (see PubMed) 60 6.8-42 Chapter 6.8: Tuberculosis 61 Duncan K., op.cit., p.206; GATB Oct 2003 internal paper, op.cit Surveillance of TB in Europe, Euro TB: Report on TB cases notified in 2001; KNCV ; Fig. 5, p.39 Accessed Feb ’04 at http://www.eurotb.org/rapports/2001/full_report_2001 62 Task force on communicable disease control in the Baltic Sea Region, Council of the Baltic Sea States. Accessed Feb 2004 at http://www.baltichealth.org/balticproject/project.php?id=43 63 Global Alliance for TB Drug Development (GATB); Economics of TB Drug Development. Oct 2001, Executive Summary, p.8 64 65 GATB; Oct 2001, op.cit., p.16 66 GATB; Oct 2001, op.cit., p.16 67 GATB; Oct 2001, op.cit., p.16 68 GATB; Oct 2001, op.cit., p.18 6.8-43