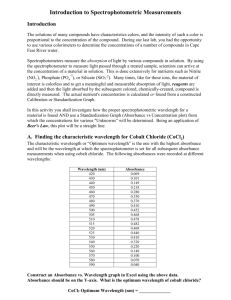
determination of maximum absorbance of a cobalt solution
advertisement

Determination of the Concentration of a Cobalt Solution Introduction: Every colored solution absorbs various amounts of light at different wavelengths of the spectrum. Before doing a spectral analysis, the optimum wavelength must be determined. This wavelength corresponds to the wavelength of light that is absorbed the most (or transmitted the least) by the sample. The first part of this experiment is to detail the procedure to be used to find the wavelength of maximum absorbance for a cobalt solution. This process involves recording the absorbance or percent transmittance (%T) over the range of 400 nm to 650 nm, usually in intervals of 10 to 15 nm. The data can be graphed either manually or by a computer program to visualize the highest absorbance or lowest %T. The wavelength of maximum absorbance is typically used to analyze the standards and unknowns in Part 2 because the instrument will be more sensitive to changes in absorbance at that wavelength. The second part of this experiment involves creating a standard curve, which will be used to determine the concentration of the unknown solution. To generate the standard curve, absorbances will be obtained for five standard solutions. A graph of this data should yield a straight line. The absorbance for the unknown and the graph will be used to determine the concentration of the unknown solution. Purpose: The purpose of this experiment is to determine the concentration of an aqueous cobalt solution. Safety: Always wear goggles in the lab. Materials: standard cobalt solutions (0.1 – 0.5M) unknown cobalt solutions Kimwipes Spectrophotometer 20 deionized water blanks small test tube rack Procedure – Part I: Determining the Wavelength of Maximum Absorbance. 1. Turn on the spec by twisting the front left knob clockwise. Allow the spec to warm up for 15 minutes. 2. Set the mode on the spec to transmittance. With the sample compartment empty, set the transmittance to 0% by turning the left front knob. Note: Do not turn this knob again during the experiment. If you do accidentally turn this knob again, notify your instructor. 3. Set the mode to absorbance. Set the spectrophotometer to 400 nm using the large knob on top. 4. Wipe the cuvette containing the water blank with a Kimwipe to remove dirt and fingerprints. 5. Insert the water blank into the sample compartment so that the white line on the cuvette matches up with the notch in the sample compartment. Set the absorbance to 0.000 using the front right knob. 6. Wipe and properly insert the cuvette containing the 0.1M cobalt solution into the sample compartment. Record the absorbance of the cobalt solution on the data sheet provided. 7. Reset the wavelength to 410 nm. Zero the instrument with the blank as in step 5. Next, record the absorbance of the cobalt solution at that wavelength as in step 6. Note: If you refrain from fingering the cuvettes except at the very top, it is not necessary to wipe the cuvettes each time. Be sure to use the 0.1M Cobalt solution for all of Part I. 8. Repeat step 7 at 10 nm intervals up to 650 nm. Be sure to zero the blank for each new wavelength, and record the absorbance on the data sheet provided. 9. After all the readings are recorded, graph the data with wavelength on the x-axis and absorbance on the y-axis. 10. When plotted, the highest peak is the wavelength of maximum absorbance. This is the wavelength that should be used in Part 2. Data – Part I: Wavelength 400 nm 410 nm 420 nm 430 nm 440 nm 450 nm 460 nm 470 nm 480 nm 490 nm 500 nm 510 nm 520 nm Absorbance __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ Wavelength 530 nm 540 nm 550 nm 560 nm 570 nm 580 nm 590 nm 600 nm 610 nm 620 nm 630 nm 640 nm 650 nm Maximum absorbance of the cobalt solution = Absorbance __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ __________ nm Procedure – Part II: Determination of the Concentration of a Cobalt Solution. 1. Set the wavelength to the wavelength of maximum absorbance. 2. Insert the water blank and zero the absorbance. 3. Insert the 0.5M cobalt solution and record the absorbance. Note: If the absorbance is off scale, repeat steps 2 and 3 with a wavelength 10 nm less than the maximum. Check several wavelengths at 10 nm intervals if necessary to bring the absorbance on scale. 4. Record the absorbance of the other standards and the unknowns at this same wavelength. (It is not necessary to zero the absorbance if the wavelength is not changed.) 5. Construct a graph using concentration as the x-axis and absorbance as the y-axis. 6. Use this graph to determine the concentration of the unknown solution. Data – Part II: Wavelength used: ________ nm Concentration 0.5M 0.4M 0.3M 0.2M 0.1M Unknown ____ Unknown ____ Absorbance Calculations: 1. Determine the concentrations of the unknown solutions. 2. Determine the percent error of your results. Questions: 1. What is the general relationship between absorbance and %T? Explain. 2. Why is it necessary to wipe the cuvettes? 3. Why is the wavelength of maximum absorbance used to analyze chemicals? 4. When might the wavelength of maximum absorbance not be used to analyze a chemical?