Unit 6: March 2
advertisement

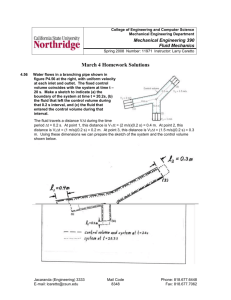
College of Engineering and Computer Science Mechanical Engineering Department Mechanical Engineering 370 Thermodynamics Fall 2010 Course Number: 14319 Instructor: Larry Caretto Solutions to Unit Six Group Exercise – Unsteady Open Systems 1. A 0.3 m3 rigid tank is filled with saturated liquid water at 200oC. A valve at the bottom of the tank is opened, and liquid is withdrawn from the tank. Heat is transferred to the water such that the temperature in the tank remains constant. Determine the amount of heat that must be transferred by the time one-half of the total mass has been withdrawn. Rigid Tank V = 0.3 m3 (a) See the diagram on the right to visualize the system. (b) Why is this problem an open system? Why is this not a steady flow system? This is an open system because mass is withdrawn from the system. It is not a steady system, because the amount of mass in the system (and probably the energy as well) is changing with time. (c) Start with the general mass balance and first law for unsteady flow systems and make the necessary assumptions and simplifications to apply the first law and mass balance to this problem. The general first law equation for unsteady open system is shown below. V2 V2 m2 u gz m1 u gz Q Wu 2 2 2 1 system 2 Vo Vi 2 mo ho gzo mi hi gzi 2 2 outlet inlet We have no data for velocity or elevations to compute kinetic and potential energies. Based on past experience, we will assume that the changes in these energy terms are negligible and will ignore these terms. Also, the system under consideration here has no way to do useful work, so the work term is zero. Finally, we note that there are no flow inlets and only one flow outlet. With all these considerations we have the following first law equation for this problem. m2u2 m1u1 system Q mouthout In the general mass balance equation, shown below, we see that the left hand side is simply -mout, because there are no inlets and only one outlet. m2 m1 system mi mo mout inlet Jacaranda (Engineering) 3333 E-mail: lcaretto@csun.edu outlet Mail Code 8348 Phone: 818.677.6448 Fax: 818.677.7062 Solutions to exercise six ME 370, L. S. Caretto, Fall 2010 Page 2 Combining the two equations to eliminate mout gives the following result for the heat transfer. m2u2 m1u1 system Q m2 m1 system hout (d) Find the properties that you will need to solve the problem. We will need the energy properties, u1, u2, and hout. In addition, we will need the initial specific volume, v1, to compute the initial mass, m 1 = V/v1. Since the initial state is a saturated liquid at 200 oC, we find u1 = uf(200oC) = 850.46 kJ/kg and v1 = vf(200oC) = 0.001157 m 3/kg from Table A-4, page 914. Since the valve is draining liquid water from the tank and the temperature remains constant at the temperature of the saturated liquid, we find that the outlet enthalpy is also a saturated liquid property, hout = hf(200oC) = 852.26 kJ/kg, from the same table. We do not know what the final state in the tank is. However we can find it from the problem statement that half of the original mass leaves the tank. First, we have to find the original mass. m1 V 0.3 m 3 v1 0.001157 m 3 259.3 kg kg The problem statement that mout = m1 / 2 allows us to compute mout and m2. mout = m1/2 = (259.3 kg)/2 = 129.65 kg m2 = m1 – mout = 259.3 kg - 129.65 kg = 129.65 kg We know that the final state has a temperature of 200 oC, and we can now compute the final specific volume from the final mass and the constant tank volume. v2 3 V 0.3 m 3 0.002314 m kg m2 129.65 kg We see that this specific volume is greater than the specific volume of the saturated liquid, vf(200oC) = 0.001157 m 3/kg, but less than the volume of the saturated vapor, vg(200oC) = 0.12721 m3/kg. Thus we are in the mixed region and have to compute the quality, x2, to find the final internal energy, u2. x2 v2 v f (T2 200o C ) v g (T2 200o C ) v f (T2 200o C ) 0.002314 m 3 kg 3 0.12721 m 0.001157 m kg 3 kg 3 0.001157 m 0.3 m 3 0.0091787 kg Now we can find the final internal energy, u2. u2 u f (T2 200o C ) x2 u fg (T2 200o C ) 1743.7 kJ 866.46 kJ 850.46 kJ (0.0091787) kg kg kg Solutions to exercise six ME 370, L. S. Caretto, Fall 2010 Page 3 (e) Obtain the answer for the heat transfer. We use the results that we have found for the masses and the energy terms into our combined first law and mass balance equation to compute the heat transfer. Q m2 u2 m1u1 system mout hout (129.65 kg) 866.46 kJ kg (259.3 kg) 850.46 kJ (129.65 kg) 852.26 kJ kg kg Q = 2,308 kJ 2. A 4 ft3 rigid tank initially contains saturated water vapor at 250oF. The tank is connected by a valve to a supply line that carries steam at 160 psia and 400 oF. Now the valve is opened and steam is allowed to enter the tank. Heat transfer takes place with the surrounding such that the temperature in the tank remains constant at 250 oF at all times. The valve is closed when it is observed that one-half of the volume of the tank is occupied by liquid water. Use the steps below to find the heat transfer. (a) Draw a diagram for this system to help you visualize the overall process. The diagram for this process is shown at the right, where the dashed line is the system boundary. Here we assume that the heat transfer has the usual sign convention so that heat input is positive and heat output is negative. The initial condition inside the tank of saturated vapor is indicated by the specification that the initial quality, x1 = 1. (b) Why is this an open system? Why is this not a steady flow system? This is an open system because mass is added to the system. It is not a steady system, because the amount of mass in the system (and probably the energy as well) is changing with time. Pin = 160 psia Tin = 400oF Inflow Q Tank V = 4 ft3 T1= 250oF x1 = 1 (c) Use property data, the mass balance equation, and the statement that the final condition is where half the volume of the tank is liquid water to find the final pressure and the mass added. We are told that the final state is a liquid-vapor mixture at 250oF. This means that the final pressure must be the saturation pressure at 250oF. From the saturation tables we find this final pressure, P2 = 29.844 psia . To find the mass added we simplify the general the mass balance equation for this problem where there is only one inlet. This gives the following result. Solutions to exercise six ME 370, L. S. Caretto, Fall 2010 m2 m1 system mi mo inlet Page 4 m2 m1 min outlet The initial mass, m1, is found from knowing the initial specific volume, v1` = vg(250oF) = 13.816 ft3/lbm, and the total tank volume, V = 4 ft3. m1 V v1 4 ft 3 3 13.816 ft 0.289 lbm lbm At the final state, half the volume of the tank is liquid and half the volume of the tank is vapor at the constant temperature of 250oF. This means that the volume of liquid is 2 ft3 and the volume of vapor is also 2 ft3. The mass of each phase is then found from the volume of the phase and the specific volume of the phase. We already know vg at 250oF is 13.816 ft3/lbm.; vf at this temperature is 0.01700 ft3/lbm. Thus the mass of each phase at the final state is given by the following equations. m f ,2 mg , 2 V f ,2 v f (250o F ) Vg , 2 v g (250o F ) 2 ft 3 3 0.01700 ft 2 ft 3 3 13.816 ft 117.64 lbm lbm 0.14 lbm lbm m2 m f , 2 mg , 2 117.64 lbm 0.14 lbm 117.78 lbm From the mass-balance equation we then find the added mass as m in = m2 – m1 = 117.78 lbm – 0.289 lbm = 117.48 lbm added . (d) Start with the general first law for unsteady flow systems and make the necessary assumptions and simplifications to find the heat transfer for this problem. The general first law equation for unsteady open system is shown below. V2 V2 m2 u gz m1 u gz Q Wu 2 2 2 1 system Vo2 Vi 2 mo ho gzo mi hi gzi 2 2 outlet inlet We have no data for velocity or elevations to compute kinetic and potential energies. Based on past experience, we will assume that the changes in these energy terms are negligible and will ignore these terms. Also, the system under consideration here has no way to do useful work, so the work term is zero. Finally, we note that there are no flow outlets and only one flow inlet. With all these considerations we have the following first law equation for this problem. m2u2 m1u1 system Q minhin Solutions to exercise six ME 370, L. S. Caretto, Fall 2010 Page 5 In the general mass balance equation, shown below, we see that the left hand side is simply min, because there are no outlets and only one inlet. m2 m1 system mi mo inlet m2 m1 min outlet (e) Find the energy properties that you will need to find the heat transfer. We will need the energy properties, u1, u2, and hin. Since the initial state is a saturated vapor at 250oF, we find u1 = ug(250oF) = 1089.7 Btu/lbm. The added steam has the constant properties of the line, so hin = h(160 psia, 400oF) = 1218.0 Btu/lbm. The final state is in the mixed region. Since we have already found the masses of liquid and vapor at this state, and since the final calculation requires the calculation of the product m 2u2, we can obtain this final product as follows: m2u2 = mf,2uf,2 + mg,2ug,2 = mf,2uf(250oF) + mg,2ug(250oF) We already found ug(250oF) = 1089.7 Btu/lbm and we can find uf(250oF) = 218.54 Btu/lbm, so we can compute m2u2 as follows. m2u2 = mf,2uf(250oF) + mg,2ug(250oF) = (117.64 lbm)(218.54 Btu/lbm) + (0.14 lbm)(1087.7 Btu/lbm) m2u2 = 25,861 Btu (f) Obtain the answer for the heat transfer. We use the results that we have found for the masses and the energy terms into our combined first law and mass balance equation to compute the heat transfer. Q m2u2 m1u1 system minhin 25,861 Btu (0.289 lbm )1087.7 Btu lb m (117.49 lbm )1217.0 Btu lbm Q = -1.175x105 Btu The negative sign for the heat transfer shows that heat is actually removed from the system. Does this seem correct to you? Let’s examine the process. We start with saturated vapor at 250oF and add steam at 400oF from the line. This means that between the initial tank contents and the added steam we have a certain amount of steam that is at 250oF or higher. We end up with a mixture that is mostly liquid (by mass) at 250oF. To get to this final state, we will have to cool the initial and incoming steam. Thus the solution that the heat transfer is negative – meaning that heat is removed to cool the system – seems correct.