Metal Rod Density Lab
advertisement

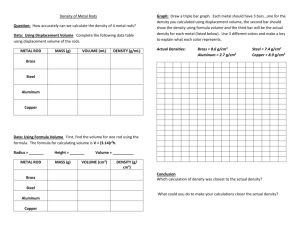
Name:________________________ Date:_________________________ Laboratory Partners:_______________________________ Measuring the Density of Different Sized Objects Materials: (4) Four Metals Rods 1 Plastic Graduated Cylinder Density Chart Lab Notebook Scale Writing instrument Calculator Procedure: 1. Fill the graduated cylinder with water about half way (this doesn’t have to be exact) and record the volume of water in milliliters (ml). 2. Weigh each metal rod individually on the scale and record the mass in grams (g) (NOTE: BE SURE THE BALANCE IS LEVEL BEFORE MEASURING). 3. After recording a metal rod’s mass slightly tip the graduated cylinder to the side and slide the rod down the cylinder into the water (NOTE: DO NOT DROP THE ROD STRAIGHT DOWN INTO THE GRADUATED CYLINDER – IT MAY BREAK (if using glass) OR SPLASH THE WATER). 4. Make sure the metal rod is completely submerged under water. 5. Since the metal rod displaced some water the water height will have raised. Record this new volume in milliliters (ml). 6. The difference between the original volume and the final volume will give you the volume of the metal rod. By knowing the rod’s volume (V) and mass (m) you can calculate the density. 7. Repeat the process for all of the other metals rods. 8. After obtaining a density for each rod, compare the values with the density table provided and make an educated guess as to what they are made of. Data Chart Mass of Metal Rod (g): Volume of Water Without Metal Rod (ml): Volume of Water With Metal Rod (ml): Density of Metal Rod (g/cm3): 1 2 3 4 Observations: Metal Bar 1: Metal Bar 2: Metal Bar 3: Metal Bar 4: Hypothesis/Results Metal Bar 1 Metal Bar 2 Metal Bar 3 Metal Bar 4 Hints: Density has units of mass (m) per volume (V), density = m/V 1 ml = 1 milliliter = 1 cubic centimeters = 1 cm3 (units of volume) The scales measure the mass of the object in grams.