Alignment layer formation and structure, FT
advertisement

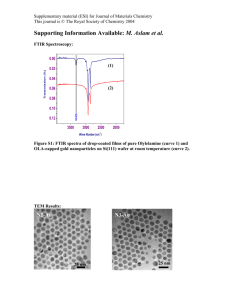
# Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 Supplemental info for “Novel alignment technique for LCD-biosensors” Additional Experimental techniques: The PI-ITO plate with the drop of buffer is placed in a Petri dish, after which a pipette is used to blow the drop from one end of the substrate to the other under an angle of 45 ° (see: Li et al, Nucleic Acids Research, 1998, 26(20), 4785-4786). The nitrogen flow is adjusted so that the drop traverses the 1,5 cm long plate in about 5 seconds. After the drop has reached the far side of the plate, the Petri dish is turned 180 degrees and the process is repeated until the drop has dried. Twisted-nematic LCD with a directionally dried TE-covered plate (direction of blowing: topbottom), with a PI-counter plate rubbed in the left-right direction (as indicated by the arrow). The image on the left is viewed between crossed polarisers, the one on the right between parallel polarisers. The anchoring energy between the TE-layer and the LC is not sufficiently high to disrupt LC-ordering induced by the PI-plate. Image is 1,0 X 0,8 cm. AFM (left) and SEM (right) images of a TE-covered PI-ITO plate made by directional drying. The arrow indicates the direction of blowing. The AFM image is 3 X 2.5 m, the bar in the SEM image is 100 nm. # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 SEM image of DNA on TE-buffer layer # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 FT-IR spectra Note: directional drying was maintained for the indicated times. 95.2 90 85 80 75 %T 70 65 3335.24 60 3188.67 55 50 45.4 3336.21 3601.5 3400 3200 3000 2800 2600 2487.1 cm-1 OH- and NH-region of a directionally dried TE-buffer in H2O. Determining the exact water content for the transition observed in these experiments (by weighing) proved difficult, gave irreproducible results and was not attempted further. Black: 5 minutes, blue: 10 minutes, red: 15 minutes. 102.5 100 95 90 85 1296.26 80 %T 75 1057.48 1038.25 70 65 60 1059.94 55 53.0 1299.5 1250 1200 1150 1100 1050 cm-1 1000 950 Ether-region (C-O and C-N) of a directionally dried TE-buffer in H2O. Black: 5 minutes, blue: 10 minutes, red: 15 minutes. 900 850 809.2 # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 106.4 100 95 1518.25 1655.16 1648.97 1284.74 90 1502.97 1477.83 %T 1546.84 1513.39 1535.28 1529.45 1624.90 85 1618.14 1358.94 1334.27 1439.11 1421.07 1467.72 1365.74 1407.47 1296.26 1447.47 1496.50 1489.72 1464.00 1435.99 1472.29 1516.16 1457.01 1417.98 80 1646.56 1616.01 1533.01 1568.99 75 1652.94 1627.081622.90 1575.85 1634.83 69.5 1673.5 1650 1600 1339.56 1521.05 1540.00 1558.63 1550 1506.95 1394.93 1386.97 1373.95 1500 1450 cm-1 1400 1350 1362.38 1300 1238.0 Carbonyl region of directionally dried TE-buffer in H2O (red) and D2O (blue). Drying time both 15 minutes. Key shifts induced by the change of the solvent: 1540.00→1535.28 1533.01→1529.45 1521.05→1518.25 1516.16→1513.39 1506.95→1502.97 93.1 90 85 80 2943.19 2889.40 75 %T 70 65 3338.02 2978.58 2943.60 60 3188.67 55.9 3645.8 3400 3200 3000 2800 2600 cm-1 OH- and NH-region of directionally dried TE-buffer in H2O (red) and D2O (blue). Drying time both 15 minutes. 2450.2 # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 16.5 1733.81 14 12 10 1436.51 8 Egy 1418.93 1670.22 1733.70 1716.94 6 1497.21 1497.12 1576.62 1716.72 1698.91 1684.20 1617.19 4 1698.77 1473.58 1457.38 1489.93 1521.50 1521.54 1635.97 1507.64 1558.62 1684.22 2 1436.99 1489.87 1507.65 1576.33 1670.24 1473.49 1458.18 1541.25 1652.95 1418.84 1403.51 1634.70 0 1555.14 -1.0 1747.8 1700 1650 1600 1550 1500 1450 1399.1 cm-1 Carbonyl region of a directionally dried TE-buffer from H2O (black, drying time 15 minutes) and a mixture of equimolar amounts of solid Tris-EDTA which was ground in a mortar (red). OH- and NH-region of a directionally (blue) and non-directionally (purple) dried drop of TEbuffer on a PI-ITO plate. # Supplementary Material (ESI) for Chemical Communications # This journal is © The Royal Society of Chemistry 2003 Directional drying of a 10 mM Urea solution Parallel LCDs made by directional blowing of a 10mM Urea solution show partial LC alignment (right image). Note on the stability of the alignment layer The observation that the alignment is lost after seven days can be explained in different ways. Two likely possibilities are: 1) The buffer gradually dissolves in 5CB over time. Experiments show that the same amount of buffer present on the surface can be dissolved in 1 ml of 5CB by sonicating at room temperature for 30 minutes. 2) The surface ordering is gradually lost by the thermal reshuffling of the hydrogen bond network, also possibly involving interactions with the cyano-group of the LC: SEM experiments showed that the surface order disappears within seven days, resulting in a featureless surface.”