Details of photoluminescence measurements, transient absorption
advertisement

Supplementary material (ESI) for J. Mater. Chem.
This journal is © The Royal Society of Chemistry 2003
[meso-Tetrakis(pentafluorophenyl)porphyrinato] Platinum(II) as an Efficient,
Oxidation-Resistant Red Phosphor: Spectroscopic Properties and Applications in
Organic Light-Emitting Diodes
Prof. Y. Wang
Key Laboratory for Supramolecular Structure and Spectroscopy of Ministry of
Education, Jilin University, Changchun 130023 (China)
Details of photoluminescence measurements; transient absorption spectra of PtF20TPP;
photophysical properties of PtF20TPP in different solvents; EL spectra and performances of
OLEDs using PtF20TPP as emitters at various doping levels; crystallographic data for
PtF20TPP.
SI 1
Supplementary material (ESI) for J. Mater. Chem.
This journal is © The Royal Society of Chemistry 2003
0.075
10s
20s
40s
80s
150s
0.050
0.025
A
0.000
-0.025
-0.050
-0.075
-0.100
-0.125
400
450
500
550
600
650
700
Wavelength (nm)
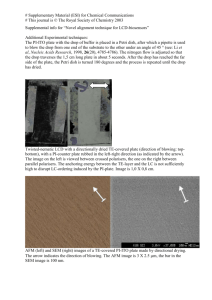
Transient absorption spectra for PtF20TPP in acetonitrile following 355 nm excitation
SI 2
Supplementary material (ESI) for J. Mater. Chem.
This journal is © The Royal Society of Chemistry 2003
Table. Photophysical Properties of PtF20TPP in different solvents
Solvent
em (nm)
(s)
Hexane
647
0.081
52
CH2Cl2
648
0.088
60
Acetonitrile
647
0.084
55
MeOH
647
0.045
26
SI 3
Supplementary material (ESI) for J. Mater. Chem.
This journal is © The Royal Society of Chemistry 2003
600
EL Intensity (a. u.)
500
10 V
400
300
5V
200
100
0
400
450
500
550
600
650
700
750
650
700
750
Wavelength (nm)
600
11 V
EL Intensity (a. u.)
500
400
300
5V
200
100
0
400
450
500
550
600
Wavelength (nm)
EL spectra of Devices 2 (top) and 4 (bottom) at various voltages
SI 4
Supplementary material (ESI) for J. Mater. Chem.
This journal is © The Royal Society of Chemistry 2003
800
700
Device 1 (4% PtF20TPP)
Device 2 (6% PtF20TPP)
Device 3 (8% PtF20TPP)
Device 4 (12% PtF20TPP)
EL Intensity (a.u.)
600
500
400
300
200
100
0
400
450
500
550
600
650
Wavelength (nm)
EL spectra of Devices 14 recorded at 8 V
SI 5
700
750
Supplementary material (ESI) for J. Mater. Chem.
This journal is © The Royal Society of Chemistry 2003
200
16
14
150
2
Luminance (cd/m )
2
Current (mA/cm )
12
10
100
8
6
50
4
2
0
0
2
4
6
8
10
12
14
Voltage (V)
140
28
120
24
80
16
60
12
40
8
20
4
0
0
4
6
8
10
12
2
Luminance (cd/m )
20
2
Current (mA/cm )
100
14
Voltage (V)
Current-voltage and luminance-voltage characteristics of Devices 2 (top) and 4 (bottom)
SI 6
Crystal Data for PtF20TPP
Crystal data: Formula: [PtF20TPP](H2O)0.33, [C44 H8.67 F20 N4 O0.33 Pt]; formula weight =
1173.64, rhombohedral, R -3, a = 14.2727(16) Å,= 88.309(17)°,V = 2903.8(6) Å3, Z = 3,
Dc = 2.013 g cm-3, (Mo-K) = 3.764 mm-1, F(000) = 1684, T = 301 K.
Data collection: A crystal of dimensions 0.4 × 0.3 × 0.1 mm mounted in a glass capillary was
used for data collection at 28oC on a MAR diffractometer with a 300 mm image plate
detector using graphite monochromatized Mo-K radiation ( = 0.71073 Å). Data collection
was made with 2o oscillation steps of , 300 seconds exposure time and scanner distance at
120 mm. 100 images were collected.
Data reduction: The images were interpreted and intensities integrated using program
DENZO 1 .
Structure solution: The structure was solved by direct methods employing SHELXS-97
program2 on PC. Almost all atoms except the water molecule were located according to the
direct methods. The position of the O atom was found after successful refinement by fullmatrix least-squares using program SHELXL-97 3 on PC.
Structure refinement: According to the SHELXL-97 program,3 all 3553 independent
reflections (Rint 4 equal to 0.0697, 2780 reflections larger than 4(Fo)) from a total 19190
reflections were participated in the full-matrix least-square refinement against F2. These
reflections were in the range -17<=h<= 17, -17<=k<= 17, -15<=l<= 15 with 2 max equal to
51.06o.
One crystallographic asymmetric unit consists of one half of formula unit, inclusive one third
of water molecule. In the final stage of least-squares refinement, all non-hydrogen atoms
were refined anisotropically. H atoms except those on O of water molecule are generated by
program SHELXL-97. The positions of H atoms are calculated based on riding mode with
thermal parameters equal to 1.2 times that of the associated C atoms, and participated in the
calculation of final R-indices5.
Convergence (( /)max = 0.001, av. 0.001) for 315 variable parameters by full-matrix leastsquares refinement on F2 reaches to R1 = 0.0336 and wR2 = 0.0848 with a goodness-of-fit of
0.994, the parameters a and b for weighting scheme are 0.0639 and 0.0. The final difference
Fourier map shows maximum rest peaks and holes of 0.482 and -1.037 eÅ-3 respectively.
Drawing: The ORTEP 6 drawing of the molecule was made with thermal ellipsoids at the 30
% probability level.
Otwinowski, Z. and Minor, W., “Processing of X-ray Diffraction Data Collected in Oscillation Mode”,
Methods in Enzymology, Volume 276: Macromolecular Crystallography, part A, p. 307-326, 1997. Carter C.
W., Sweet Jr. & R. M., Eds., Academic Press.
2
SHELXS97, Sheldrick, G. M. (1997). SHELX97. Programs for Crystal Structure Analysis (Release 97-2).
University of Goetingen, Germany.
3
SHELXL97, Sheldrick, G. M. (1997). SHELX97. Programs for Crystal Structure Analysis (Release 97-2).
University of Goetingen, Germany.
4
Rint = | Fo2- Fo2(mean) | / [ Fo2]
5
Since the structure refinements are against F2, R-indices based on F2 are larger than (more than double) those
based on F. For comparison with older refinements based on F and an OMIT threshold, a conventional index
R1 based on observed F values larger than 4(Fo) is also given (corresponding to Intensity2(I)). wR2 = { [
w( Fo2– Fc2)2] / [ w(Fo2)2] }1/2, R1 = ||Fo| – |Fc|| / |Fo|, The Goodness of Fit is always based on F2:
GooF = S = { [ w( Fo2– Fc2)2] / ( n– p) }1/2, where n is the number of reflections and p is the total number
of parameters refined. The weighting scheme is: w = 1/[2(Fo2) + (aP)2+ bP ], where P is [ 2 Fc2+ Max( Fo2,0)
]/3.
6
ORTEP3 for Windows - Farrugia, L. J. (1997) J. Appl. Cryst. 30, 565.
1
SI 7