Information Sheet for Patients in Clinical Research Project
advertisement

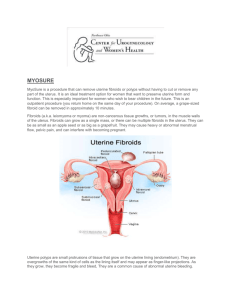
TRUST LOGO A Randomised trial of treating Fibroids with either Embolisation or Myomectomy to Measure the Effect on quality of life, among women wishing to avoid hysterectomy: the FEMME study Contact details. FEMME Study Local Organisers Doctor: Nurse: Telephone: FEMME Study Central Organisers William McKinnon FEMME Study Office, Birmingham Clinical Trials Unit, Robert Aitken Institute, Birmingham B15 2TT Tel: 0121 414 8335. email: w.mckinnon@bham.ac.uk 1 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013 Invitation to take part in the FEMME Study You have fibroids which your doctor believes can be treated equally well by either a technique called uterine artery embolisation (UAE) or conventional surgery (myomectomy). We would be grateful if you would consider taking part in our study comparing which of these procedures is best for improving the quality of life in women with fibroids. Taking part in this study is entirely up to you, and you don’t have to take part if you don’t want to. Before you decide we’d just like to explain a little more about our study, why it is being done and what taking part will involve. Please take your time to read this information sheet carefully, and feel free to talk with your friends and family about taking part. If there is anything that is not clear, or if you would like more information, you should ask your gynaecologist, interventional radiologist or the research nurse who will be delighted to answer any questions you may have. Part 1 tells you the purpose of this study and what will happen to you if you take part Part 2 gives you more detailed information about the conduct of the study Part 1: Some background information on your fibroids Fibroids are a very common, non-cancerous growth that can occur in a number of places in the womb (uterus). Being very common, most women will develop at least one fibroid at some point in their life but many will be unaware that they have one. Depending on the number, size and position of the fibroids in some women they can cause symptoms like pain during sex, heavy bleeding, abdominal pain, bloating, feeling constantly tired and a feeling of always needing to go to the toilet. Any of these symptoms can cause a significant decrease in the quality of life experienced by women with fibroids. 2 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013 What are the standard treatments for fibroids? One of the standard surgical treatments for fibroids is called hysterectomy. Hysterectomy is quite a major operation where the entire womb is surgically removed. With many women associating their womb with their femininity and sexuality, and with fibroids being a benign condition, many women are asking if they are losing their wombs unnecessarily when they have a hysterectomy. As it removes the womb, hysterectomy is not an option for women who may want to try to have children. More recently other techniques have been developed which treat the fibroid and allow the woman to keep her womb. The first of these treatments is called myomectomy. This is a procedure whereby the fibroid is removed by a variety of surgical techniques. The second treatment is called Uterine Artery Embolisation and is often called UAE. The National Centre for Health and Clinical Excellence (NICE) has issued guidelines outlining treatments for heavy menstrual bleeding (reference CG44). You can download these from NICE’s website www.nice.org.uk, or you can request them by email publications@nice.org.uk or ‘phone 0845 003 7783. What do the procedures involve? A myomectomy is a surgical procedure performed under general anaesthetic. There are three ways the surgeon can reach the fibroid: Through small holes made in the abdominal wall (Laparoscopic or keyhole surgery) Through the vagina and neck of the womb (Hysteroscopic) Through a horizontal or vertical cut in the abdomen (Open surgery) The surgeon will decide on which route to use depending on the size, number and location of your fibroids. Your surgeon will discuss this decision with you. UAE is routinely performed under local anaesthetic and is usually completed within one hour. In UAE a small tube is introduced into the blood supply to the fibroid. Once in place small beads are placed into the blood vessel which reduces the blood flow to fibroids. This causes the fibroids to shrink over a period of several months and so relieves the symptoms. When will I be able to go home and when will I be OK to return to normal activities? The recovery times are different for the two procedures. After UAE, most women can leave hospital in less than 48 hours but should rest at home for 1-2 days. The length of stay in hospital after a myomectomy depends on the procedure used and is around 2-3 days after a keyhole or hysteroscopic procedure, and 3-7 days after open surgery. These figures are guides and just how quickly you return to normal activities will depend on many factors. Your doctor will be able to give you more specific advice regarding returning to work or normal activities after your procedure. 3 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013 What is the purpose of the study? UAE and myomectomy are both safe, effective procedures that are readily available on the NHS, but doctors do not know which is best in improving the quality of life for women with fibroids. It is important to compare the two procedures against each other and our study will do this. Why have I been asked to take part? You have been asked if you would like to take part in this study as you have fibroids that your doctor believes can be treated equally well by either UAE or myomectomy. Please be assured that as it is important that you always receive the best treatment for you that if your doctor felt that one treatment would be more beneficial to you they would not have invited you to take part in this study. Do I have to take part? It is entirely up to you if you want to take part in this study. You do not have to take part if you don’t want to and you do not have to give a reason why. If you do not want to take part in this study then the standard of care you will receive will not be affected. If you do decide to take part in this study but later change your mind then you are free to withdraw from the study at any time. As before, you don’t have to give a reason why you have changed your mind and don’t want to take part, and this will not change the standard of your medical care in any way. Participation in this study will not affect any private health insurance. What will happen to me if I take part? If the doctor feels that your fibroids can be treated by UAE or myomectomy, and if you agree to take part in the trial, you will be asked to sign a consent form to say that the trial has been explained to you, that you understand what taking part in the trial will involve and that you are willing to take part. You will be asked to complete a questionnaire which will record how your fibroid is affecting your quality of life and then hand this back to the nurse. With your permission, we would like to take 10 mls (two teaspoons) of blood. This will be used to measure the levels of the hormones associated with your fertility (ovarian reserve). All your blood samples will be stored securely under a trial number at the University of Birmingham until the end of the study when they will be sent to the University of Glasgow where the hormone levels will be measured. You will not be able to see your specific results but we will give you the pooled results of all the other women who had the same operation as you did. After the study is finished we’d like to keep your blood sample for use in future studies. You will then be assigned to have either a UAE or a myomectomy. You have an equal chance of having a myomectomy or a UAE but which operation you have will be chosen at random and neither you nor your doctor can choose what it will be. As your doctor will always act in your best interest this is why they must feel that your fibroids will respond equally well to both myomectomy or UAE. As the treatment is always chosen at random this also means that if you have a preference for the treatment you’d like to receive then you won’t be able to take part in the trial. At the end of the day it is your body and you must be happy with the treatment you receive. 4 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013 If you have not already had one, you may have a magnetic resonance imaging scan (MRI) to get a more detailed picture of the fibroid(s) in your uterus. The scan is completely painless and there are no known risks or side-effects. You will be asked to lie still in the scanner for 10-15 minutes. The scanner can be very noisy and hearing protection will be given. You will be able to contact the radiographer at all times during the scan if you need assistance. A small injection of special dye will be given into one of the veins in your arm. If I take part in the FEMME study, what else will happen after the procedure? You will have a routine check-up at 6 weeks after your myomectomy or UAE procedure. At 6 months, you may be invited to return for a follow-up MRI scan to see how successful the treatment has been and you will be asked to fill in the same questionnaires you completed before the procedure. The same questionnaires will be sent to you at home at 1, 2 and 4 years after your operation. This will allow us to measure how your quality of life changes over time and see which treatment is best to offer to women with fibroids. To be as certain as possible that our answer is correct it is important to find out how all women who are taking part are progressing so the study organisers may telephone, text or email you to remind you to complete the questionnaires. We would like to repeat your blood test at 6 weeks, 6 months and 12 months after your operation to see if your procedure has affected the levels of hormones associated with ovarian function. Should you get pregnant at any time after the procedure, we would like you to contact the Trial Office. We would like to collect a few details about the outcome of the pregnancy, which we will do by telephone or through your GP. What are the risks and discomforts? Myomectomy and UAE are both safe procedures and are in routine use within the NHS. Some studies have suggested that women who have a UAE recover from their operation more quickly than those who have a myomectomy, but may be more likely to have the need for more treatment in the future. On top of this, both myomectomy and UAE are associated with a range of complications. Although rare the risks of each procedure can include: Myomectomy • • • • • • • Haemorrhage Injury to the uterus Damage to the nearby organs of the urinary system Formation of scar tissue (adhesions) within the uterus Infection Blood clots Eventual re-growth of fibroids. The effect that myomectomy has on the chance of getting pregnant is unknown at present. UAE • Damage to the uterus, bladder, vulva and ovaries 5 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013 • • • • Flu-like symptoms Pain Vaginal discharge Early menopause. The effect that UAE has on the chance of getting pregnant is unknown at present. In order to ensure that the tube delivering the beads is in the right place it will be guided there under x-ray imaging. Whilst any exposure to ionising radiation carries an increased risk of developing cancer, it is the size of this risk that is important. The lifetime risk of developing a fatal cancer following UAE is 1 in 3,330. This is similar to the lifetime risk of dying from an accident at work in the manufacturing industry, and a lot less than the lifetime risk of dying in a transportation accident in the UK (1 in 240). Your doctor will discuss the risks and discomforts of the allocated treatment and give you more details of what to do if you experience any side effects. Are there any benefits for me from taking part in the study? You will not gain any individual benefit by taking part in the study but the trial will provide valuable information to decide which treatment is best for future women suffering from fibroids. What if there is a problem? Any complaint about the way you have been dealt with during the study or any possible harm you might suffer will be taken very seriously, thoroughly investigated and any matters arising will be addressed. Detailed information on who to contact to raise a concern is given in Part 2 (What if there is a problem?). If the information in Part 1 has interested you and you are considering taking part, please read the additional information in Part 2 before making any decisions 6 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013 Part 2: Conduct of the study What will happen if I don’t want to carry on with the study? If you change your mind and decide that you do not want to continue taking part in the study you can withdraw at any time. You don’t have to give a reason why you no longer wish to take part, and withdrawing will not affect the standard of your medical care in any way. If you do decide that you no longer wish to take part then we would like to use the information collected about you up to the point of your withdrawal. In the unlikely event of you losing the ability to give continued consent during the study we would like to keep data that we have already collected about you and include it in our study. Will information about me be kept confidential? Yes. Like your medical records all information collected in the study will be held securely and remain strictly confidential. If you agree to take part, your doctor will send basic information about you and your condition to the study’s central organisers at the University of Birmingham’s Clinical Trials Unit. This information will be put into a computer and assigned a code number. The answers you give be will be identified using this code number, not your name. Any information you give will not be seen by your GP or gynaecologist. No information from which you can be identified will be published in the study report. Information held by the NHS may be used to keep in touch with women taking part in the study and follow up their health status. Occasionally, inspections of clinical study data are undertaken to ensure that, for example, all participants have given consent to take part, so a copy of your consent form will be sent to the FEMME study office. Selected members of bodies responsible for good conduct of the trial from the University of Oxford or the NHS Trusts may also be given access to data to ensure we are complying with regulations. But, apart from this, only a very small number of study organisers will have access to your personal data. Involvement of the General Practitioner/Family doctor (GP) With your consent we will inform your GP of your participation in the FEMME Study. Your GP will not be able to access your answers or any of the information we hold on you. Even if you no longer wish to complete the questionnaires, we would like to continue to collect a few important details from your GP, such as information on repeat surgeries, if you get pregnant and the outcome of this pregnancy. What will happen to the results of the research study? The results will be reported in a medical journal. It is expected that the first results will be published about two years after the study finishes recruiting women. Everyone who takes part will then be told the results in a newsletter that will be posted directly to them. Who is funding and organising the research? The FEMME study researchers are receiving a grant from the National Institute for Health Research’s Health Technology Assessment programme (NIHR HTA) to enable them to carry out this study. 7 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013 The study is being sponsored by the University of Oxford, co-ordinated from Birmingham Clinical Trials Unit and being run with significant input from clinical staff based in Glasgow (Royal Infirmary) and London (St. George’s Hospital). No one involved is being paid for recruiting women into the study. Patients are not paid to take part either, but their help in finding out more about how best to treat fibroids is very much appreciated. Who has reviewed the study? All research in the NHS is overseen by an independent group of people called a Research Ethics Committee (REC) whose role is to ensure your safety, rights, wellbeing and dignity at all times. This study has been reviewed and given a favourable opinion by the Coventry and Warwickshire REC. This study has also been reviewed by the Research and Development (R&D) Department for each hospital trust taking part in the trial. What if there is a problem? The study is being sponsored by the University of Oxford who have arrangements in place to provide compensation in the extremely unlikely event that you suffer lasting harm from participation in the study. NHS indemnity operates in respect of the clinical treatment you receive. You have the same legal rights whether or not you take part in this study. If you wish to complain about any aspect of the way in which you have been approached or treated during the course of this study you should initially contact the local investigator named on the cover of this information sheet. If you are unhappy with their response then you should raise your concern with the University of Oxford’s Clinical Trials and Research Governance (CTRG) office on 01865 857939 or by email (heather.house@admin.ox.ac.uk). Do you have any further questions? Having read this leaflet, we hope that you will choose to take part in the FEMME Study. If you have any questions about the study now or later feel free to ask your gynaecologist or clinic nurse. Their names and telephone numbers are given on the front of this leaflet. Please take the time to decide whether you wish to take part in the FEMME Study. Please feel free to discuss your decision with friends or relatives. If you require any general information about research the UK Clinical Research Collaboration has produced a useful guide entitled, ‘Understanding Clinical Trials’. This can be downloaded from their website: www.ukcrn.org.uk. If you require specific information about the research project please either contact any of the FEMME staff listed on the front page or visit our website: www.birmingham.ac.uk/femme. Only the clinical staff can give you advice about your medical conditions and the surgical options that may be available to you. Thank you for taking the time to read this Participant Information Sheet about the FEMME Study. 8 Coventry & Warwickshire REC/ Sponsor No: 11/WM/0149 ISRCTN70772394 FEMME Participant Information Sheet (Blood Centres) Version 1.5 04th November 2013