Plasmid constructions - Springer Static Content Server
advertisement

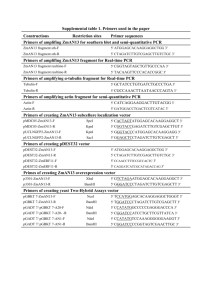
Plasmid constructions The plasmids used in this study are listed in Table 1. All DNA manipulations followed standard procedures (Sambrook et al. 1989). For the construction of the gene encoding the TorA-GFP fusion protein for analysis in S. carnosus, a torA gene fragment encoding the signal peptide of the E. coli TorA protein was amplified by PCR using plasmid pXR100TorA-CGT (A.Vollstedt, unpublished) as a template and primers TSc-for (5′-CTC TAG AGT CGA CCT AGA GTC GAT-3′) and TSc-rev (5´-CGC CGC TTG CGC CGC AGT-3′). A gfp gene fragment without start codon was amplified using the vector pGFPuv (Clontech) as a template and primers GSc-for (5´-ACT GCG GCG CAA GCG GCG CGT AAA GGA GAA GAA CTT TTC ACT GGA -3´) and GSc-rev (5´-CGC CAT GCT GGA ATT CAT TAT TTG TAG AGC TC-3´). Both fragments were purified and used as a template in a cross-over PCR reaction using primers TSc-for and GSc-rev. The resulting PCR fragment containing the torAgfp gene was digested with XbaI and SphI, and ligated into the XbaI/SphI-digested vector pXR100 (Sandgathe et al. 2003), resulting in pSCTorA-GFP. For expression of torA-gfp in B. subtilis, the torA-gfp gene fragment was amplified by PCR using the plasmid pSCTorA-GFP as a template and primers TBs-for (5′-GAT GGT ACC GAT CTA GAA CTA GTG GAT C 3′) and GBs-rev (5´-CGC GCA TGC TGG AAT TCA TTA TTT GTA GAG CTC -3′). The resulting PCR fragment was digested with KpnI and SphI, and ligated into the SphI/XbaIdigested vector pWH1520 (Rygus et al. 1991), resulting in plasmid pBSTorA-GFP. For expression of torA-gfp in C. glutamicum, the torA-gfp gene was amplified by PCR using plasmid pSCTorA-GFP as a template and primers TCg-for (5′-GGC CTG CAG AAG GAG ATA TAG ATA TGA ACA ATA ACG ATC TCT TTC AGG CAT C -3′) and GCg-rev (5´CAG GAA TTC ATT ATT TGT AGA GCT CAT CCA TGC CAT G -3′). The resulting PCR fragment was digested with PstI and EcoRI, and ligated into the PstI/EcoRI-digested vector pEKEx2 (Eikmanns et al. 1991), resulting in plasmid pCGTorA-GFP. For the construction of the gene encoding the PhoDCg-GFP hybrid precursor, the phoDCg DNA fragment (encoding the signal peptide of the C. glutamicum PhoD phosphodiesterase) was amplified by PCR using chromosomal DNA of C. glutamicum ATCC13032 as a template and primers PCg-for (5′-GGC GGT ACC AAG GAG ATA TAG ATA TGC CAC AGT TAA GCA GAC GCC AGT TC-3′) and PCg-rev (5´-AGT TCT TCT CCT TTA CGT TGG CGT TCT TCA GCG CGT GCA G -3′). A gfp gene fragment without start codon was amplified using the vector pGFPuv as a template and primers GPCg-for (5´-GTA AAG GAG AAG AAC TTT TCA CTG GAG -3´) and GPCg-rev (5´-CAG GAA TTC ATT ATT TGT AGA GCT CAT CCA TGC CAT G -3´). Both fragments were purified and subsequently used as template in a crossover PCR reaction using primers PCg-for and GPCg-rev. The resulting PCR product containing the phoDCg-gfp gene was digested with KpnI and EcoRI, and ligated into the KpnI/EcoRI-digested vector pEKEx2, resulting in pCGPhoDCg-GFP. For the construction of the gene encoding the PhoDBs-GFP hybrid precursor, the phoDBs DNA fragment (encoding the signal peptide of the B. subtilis PhoD phosphodiesterase) was amplified by PCR using chromosomal DNA of B. subtilis 168 as a template and primers PBs-for (5´-GAG GTA CCA TGA GGA GAG AGG GGA TCT TGA ATG-3′) and PBs-rev (5′-AGT TCT TCT CCT TTA CGG AAG TTA GGC GCA GCA TTT ACT TC-3′). The gfp gene fragment without start codon was amplified using the vector pGFPuv as a template and primers GPCg-for and GScrev. Both fragments were purified and subsequently used as template in a cross-over PCR reaction using primers PBs-for and GSc-rev. The resulting PCR product containing the phoDBs-gfp gene was digested with KpnI and SphI, and ligated into the KpnI/SphI-digested vector pWH1520, resulting in pBsPhoDBs-GFP. Subsquently, the phoDBs-gfp gene was amplified by PCR using the vector pBsPhoDBs-GFP as template and primers GPBs-for (5´GAG CTG CAG AAG GAG ATA TAG ATA TGG CAT ACG ACA GTC GTT TTG ATG3′) and GCg-rev. The resulting PCR fragment was digested with PstI and EcoRI, and ligated into the PstI/EcoRI-digested vector pEKEx2, resulting in pCGPhoDBs-GFP. The correctness of all plasmid constructions was verified by DNA sequencing. References: Eikmanns BJ, Kleinertz E, Liebl W, Sahm H (1991) A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene 102: 93-98 Sambrook J, Fritsch EF Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, New York Sandgathe A, Tippe D, Dilsen S, Meens J, Halfar M, Weuster-Botz D, Freudl R, Thömmes J, Kula MR (2003) Production of a human calcitonin precursor with Staphylococcus carnosus: secretory expression and single-step recovery by expanded bed adsorption. Process Biochem 38: 1351-1363 Rygus T, Scheler A, Allmansberger R, Hillen W (1991) Molecular cloning, structure, promoters and regulatory elements for transcription of the Bacillus megaterium encoded regulon for xylose utilization. Arch Microbiol 155: 535-542