howdoi~2
advertisement

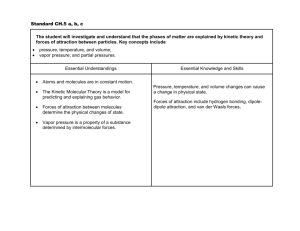
SCH3UB Date __________________________________ How do Intermolecular Forces affect boiling points and melting points? Explain each of the following in terms of the intermolecular forces that exist. a) At room temperature and pressure, chlorine is a gas, bromine is a liquid and iodine is a solid. o Cl2, Br2 and I2 are all nonpolar diatomic molecules – there is no dipole-dipole attraction possible and they are not capable of hydrogen bonding o The only type of intermolecular force of attraction that affects their boiling point is van der Waal’s forces of attraction between neighbouring molecules o Van der waal’s forces of attraction increase as a molecule’s mass increases (and # of electrons increases) o Chlorine has the smallest mass of the three halogens mentioned, so has the fewest electrons and will have the weakest van der Waal’s forces so it has the lowest boiling point because it is easiest to separate molecules of chlorine from each other; bromine has the next highest mass, so has intermediate van der Waalk’s forces and would have the next highest boiling point (above room temperature), while iodine has the greatest mass, the strongest van der Waal’s forces of attraction and remains in solid state at room temperature; b) Ethanol (CH3CH2OH) has a much higher boiling point than its isomer (same formula but a different structural arrangement of atoms) methoxymethane (CH3OCH3) ethanol o Both have the same chemical formula, same mass and same number of electrons, so will have very similar van der Waal’s forces of attraction o Methoxymethane has 2 polar C-O bonds, but they are equal and exactly opposite and cancel out to zero, so it is a nonpolar molecule and only has van der Waal’s forces of attraction, the weakest of the intermolecular forces, so it will have a fairly low boiling point as it is easier to separate molecules and overcome the weak van der Waal’s forces o Ethanol has a very polar O-H bond, so it is capable of hydrogen bonding with neighbouring molecules; hydrogen bonding is the strongest intermolecular force, so its boiling point is much higher than methoxymethane d) The boiling point of sulfur dioxide is 240C higher than that of chlorine. o Chlorine, Cl2, is a diatomic, nonpolar molecule; the only intermolecular forces of attraction between chlorine molecules is van der Waal’s forces of attraction, the weakest type of intermolecular force of attraction o SO2 is a polar molecule (bent shape – draw a Lewis structure and identify the shape) so it has dipole-dipole attractive forces between molecules that are much stronger than van der Waal’s and this makes it more difficult to separate molecules of sulfur dioxide so its boiling point is higher. o Although Cl2 has a larger mass than SO2 and so has larger van der Waal’s forces of attraction between molecules, the much stronger dipole-dipole attractive forces present between SO2 molecules make for much stronger intermolecular forces of attraction between SO2 molecules SCH3UB Date __________________________________ e) The boiling point of hydrogen fluoride, water and ammonia is significantly higher than those of the analogous compounds in the next period (period 3). For each pair, explain why: (ii) HF and HCl HF has a much higher boiling point than HCl because HF is a very polar bond and is capable of hydrogen bonding between molecules. Hydrogen bonding is the strongest type of intermolecular force of attraction. HCl has dipole-dipole forces and van der Waal’s forces of attraction between molecules, but these are weaker than hydrogen bonding and so it has a lower boiling point because it is easier to separate molecules of HCl from each other during the change from liquid to gas. (ii) H2O and H2S ( at room temperature H2O is a liquid but H2S is a gas) H2O has a much higher boiling point than H2S because O-H is a very polar bond and the water molecule is capable of hydrogen bonding between molecules. Hydrogen bonding is the strongest type of intermolecular force of attraction. H2S has dipole-dipole forces and van der Waal’s forces of attraction between molecules, but these are weaker than hydrogen bonding and so it has a lower boiling point because it is easier to separate molecules of H2S from each other during the change from liquid to gas. (iii) NH3 and PH3 NH3 has a much higher boiling point than PH3 because N-H is a very polar bond and the ammonia (NH3) molecules are capable of hydrogen bonding between molecules. Hydrogen bonding is the strongest type of intermolecular force of attraction. PH3 has dipole-dipole forces and van der Waal’s forces of attraction between molecules, but these are weaker than hydrogen bonding and so it has a lower boiling point because it is easier to separate molecules of PH3 from each other during the change from liquid to gas. SCH3UB Date __________________________________