Chemical Reactions
Chemical Reactions Homework 2
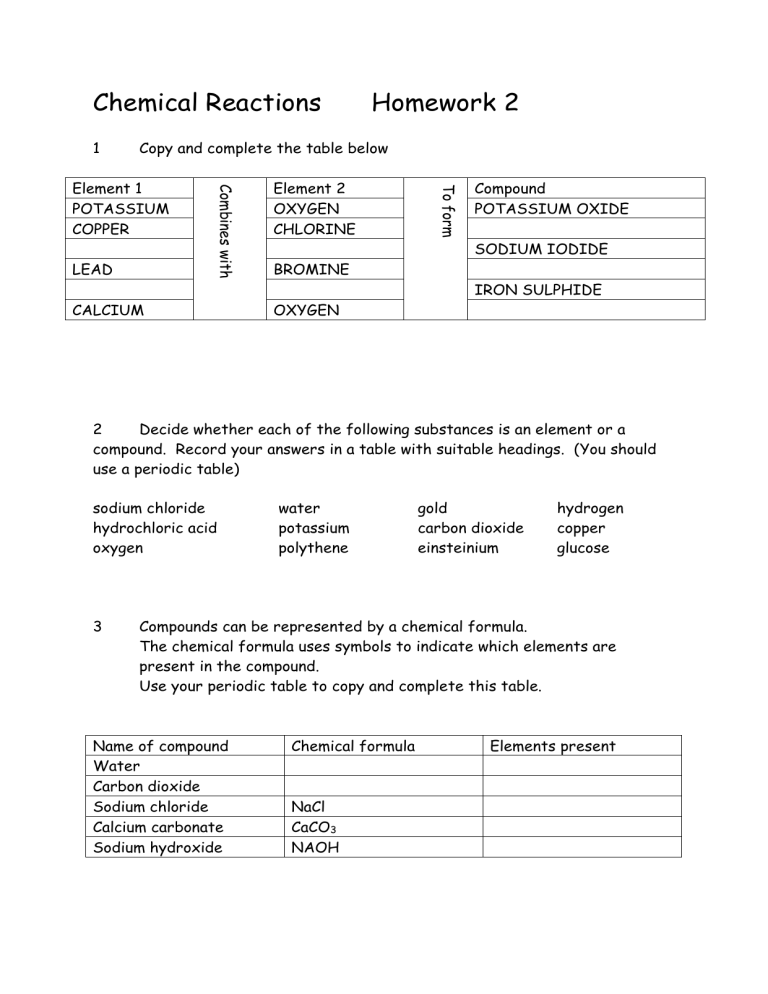
1 Copy and complete the table below
Element 1
POTASSIUM
COPPER
Element 2
OXYGEN
CHLORINE
Name of compound
Water
Carbon dioxide
Sodium chloride
Calcium carbonate
Sodium hydroxide
Compound
POTASSIUM OXIDE
LEAD
CALCIUM
BROMINE
OXYGEN
SODIUM IODIDE
IRON SULPHIDE
2 Decide whether each of the following substances is an element or a compound. Record your answers in a table with suitable headings. (You should use a periodic table) sodium chloride water gold hydrogen hydrochloric acid oxygen potassium polythene carbon dioxide einsteinium copper glucose
3 Compounds can be represented by a chemical formula.
The chemical formula uses symbols to indicate which elements are present in the compound.
Use your periodic table to copy and complete this table.
Chemical formula
NaCl
CaCO
3
NAOH
Elements present
Chemical Reactions Homework 3
1 a) There are many signs of a chemical reaction. One of these is
precipitation. Describe what happens in a precipitation reaction. b) Bubbling and fizzing was seen during the reaction. What word is used to describe “bubbling and fizzing”?
2 Chemical reactions take place at different rates. Put the following reactions in order starting with the slowest one first. a cake baking an explosion iron rusting milk turning sour
3 The rate of a chemical reaction can change by altering the conditions.
What effect will changing the following things have on the rate of reaction? a) Increasing the temperature. b) Using lumps of chalk instead of powdered chalk in a reaction with acid. c) Keeping milk in a fridge at 0oC instead of 10oC. d) Cutting up potatoes into smaller cubes before boiling.
Chemical Reactions Homework 4
1 When magnesium acid is added to hydrochloric acid (concentration 4 mol/l) at 25oC the reaction is very fast.
What changes could be made to slow the reaction down?
2
3
What is a CATALYST?
Decide whether or not each of the following IS or IS NOT a chemical reaction. a) Mixing two solutions to produce a gas. b) Water boiling in a kettle. c) Mixing two solutions to form a yellow solid. d) Mould growing on a piece of bread. e) Using a magnet to separate iron from a mixture. f) Wool growing on a sheep g) A gas explosion. h) Skin forming over a cut i) Breaking glass j) Paint drying on a fence.
4 The chemical sodium hydroxide is an alkali which is widely used in industry.
50% of all sodium hydroxide is used to make textiles and paper.
10% is used to make soap
10% is used to make aluminium
30% is used in water treatment
Put this information into a table AND a bar graph.
Chemical Reactions Homework 1
1
A B C D
2
Select which diagram above (A,B,C or D) shows….. a) a compound b) an element c) a mixture of two compounds a) Approximately how many elements are there in existence? b) The elements of the periodic table are divided into two main types, what are they?
3 A pupil heated a mixture of the elements IRON and SULPHUR. A chemical reaction took place in which a COMPOUND was formed. a) Explain the difference between a COMPOUND and an ELEMENT. b) What is the name of the compound that forms in the reaction between sulphur and iron? c) What things might you see / hear to indicate that a reaction had taken place? d) If the mixture of iron and sulphur had not been heated there would not have been a chemical reaction. How could the iron and sulphur be separated? e) The iron and sulphur in the new compound cannot be separated easily,
Why is this?










