Lesson Plan
advertisement

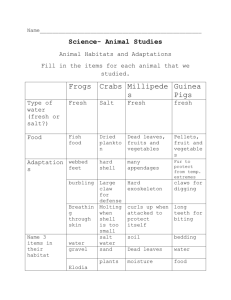
DOUBLE DIFFUSION: SALT FINGER PURPOSE: To show the combined effect of competing physical processes, temperature and saltiness, that can cause mixing of different types of seawater. INTRODUCTION: Physical oceanographers examine the ways ocean water mixes. It’s important because this jumbling helps seawater move around or circulate. This flow in the seas contributes to weather and climate (longterm weather), which in turn affect plants and animals in the sea, in the air above it, and on the nearby land. By churning up the sea at depth and sideways, nutrients (food and minerals) can be brought to the sea plants, and then to the animals that graze on them, so that all the living things in this marine environment can continue to live. One basic physical process moving water about is heating. Heat causes the water to expand, thus making it less dense (density is weight per unit volume, grams/cu cm). Nearby unheated water, colder, is therefore more dense and by gravity pushes the less dense water aside and up. Another fundamental way to move water is to change its density by how much salt it has in it. The more salt it has, the greater its density. Thus, density differences can move water, whether they are based on temperature, salinity or both. This demonstration looks at both processes occurring at once so we call it double. And because the fluids do spread, we call it diffusion. Our demonstration is that of double diffusion instabilities. MATERIALS: 2 glasses of same size and style Table salt, NaCl Phenolphthalein Sodium hydroxide, NaSO4 Index cards PROCEDURE: 1) Mix in glass the upper layer: really hot water from the tap, an eyedropper squirt of the 1% phenolphthalein indicator in alcohol, and ~1/16 teaspoon of salt, NaCl (get close to a .1% by volume solution). FILL IT FULL. 2) Mix in the second glass the lower layer: very cold water with ~1/32 teaspoon of sodium hydroxide, NaOH. FILL IT TO THE BRIM. 3) Take the index card and place it over the mouth of the hot/salty water glass. Slowly invert the bottle upset down gently holding the card in place. 4) Position the card and glass directly on to the open mouth of the lower or cold/”fresh”(disregard the small amount of sodium hydroxide put in it; for our density purposes it is essentially fresh) water glass. 5) Then as if a magician, gingerly pull out the index card, allowing the fluids to come into contact (if they were completely full, they won’t spill into one another and the diffusion rate will be ideally slow). EXPLANATION: What should happen is that little bumps of the two different solutions at the interface should start to grow. This growth causes mixing which is indicated by the phenolphthalein dye changing from clear to pink as its comes in contact with the basic sodium hydroxide solution. It’s surprising that the growth occurs both upwards and downwards. Here’s why. Suppose a little fresh cold spot bumps upward because it’s unstable. It’s unstable by having competing density conditions: the fresh water is less dense than the salty, so the fresh tends to get pushed upward; but the cold part of it makes it denser, so it stays down. Once this cold/fresh perturbation enters the overlying hot/salty area, it gets heated. This heating lowers its density. Thus, the bump “grows” upward because it’s becoming less dense. Or you can explain the changes from the point of view of the upper layer. Say a salty/hot spot pushes downward (due to density effects of the salt). It goes into the cold/fresh layer, losing heat and becoming colder. As it grows colder, it gets denser, so it goes down. (Notice that the reverse situation, cold/fresh overlying hot/salty, will not grow the salt fingers, but it will show another phenomenon, a salt staircase of layers of differing densities.) These growing interweavings as indicated by the color changes are called salt fingers. They occur between the bottom of hot, salty Mediterranean Sea outflow and cold, less salty Atlantic Ocean water beneath. All in all, the molecular diffusion rate is about 100x faster for temperature than it is for salinity. (This demonstration is taken from http;//www.atmos.Washington.edu/gfd_exp/exp_e/exp/sf/1/app.htm)