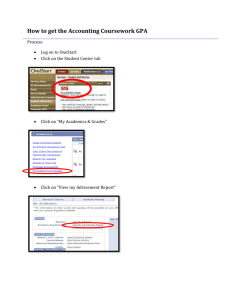
CaO as a Source Indicator
advertisement

Petrology and thermal structure of the Hawaiian plume: a view from Mauna Kea Claude Herzberg Department of Geological Sciences Rutgers University Piscataway, New Jersey, U.S.A. 08854 Herzberg@rci.rutgers.edu Supplementary Information This material provides a detailed explanation of peridotite and pyroxenite modeling. CaO Contents of Batch Melts of Fertile Peridotite The CaO contents of experimental melts as functions of melt fraction (F) and pressure1 are shown in Figure S1 for fertile peridotite KR-4003. These are compared with computed liquids in equilibrium with olivine [L+Ol] and olivine+orthopyroxene [L+Ol+Opx] from mass balance solutions to the equilibrium melting equation2. There is excellent coverage of experimental data at 6, and 7 GPa1, but fewer data at 4 and 5 GPa, especially at low melt fractions. Calculated and observed compositions at 3 GPa indicate CaO = 10% where F = 0, but additional experimental data at low melt fractions is needed for a more secure evaluation. Experimental data and computed liquids at 3, 6, and 7 GPa have been regressed to F = 0, and the results illustrate the CaO content of liquidus on the solidus is about 10%, and does not vary significantly with pressure in the 3-7 GPa (Figure S1). Results at 4 and 5 GPa are interpolations. CaO is now plotted with MgO for experimental data and computed liquids in Figure S2. Also shown, for purposes of comparison, are the approximate CaO and MgO contents of fertile peridotite at 1 and 2 GPa4-8. At pressures in the 1 to 3 GPa range, dissolution of clinopyroxene with progressive melting above the solidus causes the CaO contents of melts to increase; CaO reaches a maximum when all Cpx is melted out. CaO then drops with continued progressive melting for the assemblages [L+Ol+Opx] and [L+Ol]. However, the situation is different at pressures in the 4 to 7 GPa. CaO is at a maximum for liquids on the solidus, and drops with continued progressive melting for all assemblages above the solidus, which are typically [L+Ol+Cpx+Gt], [L+Ol+Opx+Gt], [L+Ol+Opx], and [L+Ol] (see Herzberg and O’Hara2 for a complete description of these assemblages). CaO Contents of Accumulated Fractional Melts of Fertile Peridotite A detailed discussion of the effects of perfect fractional melting on the FeO and MgO contents of Kettle River Peridotite KR-4003 was given in Herzberg & O’Hara2. Briefly, a computation of the liquid was made of 0.01 mass fractions of equilibrium melting of the residue, and the liquid was perfectly removed from its residue. This fractional melting process changes the composition of each successive residue, which is melted again at 0.01 mass fractions of melting. Each liquid formed by 0.01 mass fractions of melting is called an instantaneous melt. Each new melt was perfectly extracted and mixed with the previously formed melt, and the mixture is the aggregate fractional melt. Results of fractional melting on FeO and MgO1 were extended to include Al2O3 and SiO2 2, and computation procedures used here for CaO are the same. That is, 1 the compositions of CaO in the instantaneous melt produced at 0.01 mass fractions of melting were calculated from parameterizations of D for CaO as polybaric functions of the MgO content of the residue. These instantaneous melts mix to yield an aggregate melt, and the CaO contents for these are shown in Figure S3, and Figure 1b in the text. CaO contents of Batch Melts of Fe-rich Peridotite Melting experiments on Fe-rich peridotite with 10.3% FeOT indicate that CaO might be lowered compared to those in Figures S1 and S29 for normal peridotite having about 8% FeO. Humayun et al.10 argued that Hawaiian tholeiites might have been produced from Fe-rich mantle peridotite. Could the low CaO parental lavas from Mauna Kea be diagnostic of Fe-rich mantle peridotite? A close examination of Kushiro’s9 experiments indicates this is unlikely because, while CaO might be appropriately lowered, the SiO2 contents are consistently too low by 2-4 wt% absolute, and would yield alkalic lavas rather than the observed tholeiitic compositions. CaO Contents of Peridotite Melts used in the Analysis of Stolper et al.11 Peridotite partial melt CaO contents at 30 kilobars used in the Stolper et al.11 analysis, adopted from a parameterization of Longhi12, appear to match for CaO content of their Mauna Kea low SiO2 parental magma. However, these model peridotite melts are significantly lower in SiO2 than the parental magma compositions, and they are alkalic not tholeiitic. There is some agreement in CaO between the model low SiO2 parental magma Stolper et al.11 and experimental data at 28-32 kilobars from Salters et al.13 on liquids equilibrated with “lherzolite assemblages” (i.e., L+OlOpxCpxGt). However, those experimental melts are alkalic, not tholeiitic. The parental Kea high SiO2 parental magma of Stolper et al.11 does not match up with any experimental peridotite melts at ~ 30 kilobars. The match is improved with Longhi’s12 model peridotite melts at 1.5 GPa; however, CaO in the parental magmas remains about 1.5% lower than these peridotite melts, and garnet will not be stable at this pressure. Erroneous Peridotite Source Primary Magmas in the Models of Herzberg and Coauthors2,14 A new way to obtain model primary magma solutions was described in Herzberg & O’Hara2 and applied to the low and high SiO2 whole rocks from the HSDP2 project14. The method is described in the footnote to Table 1. It is a coupled forward and inverse model using assumed peridotite compositions. The forward component consisted of primary magma compositions in FeO-MgO and CMAS projection space, constrained by experiment and calculation on assumed peridotite source compositions. A comprehensive model for peridotite-source melting must satisfy all major elements in simple plots and in projection, a requirement of Duhem’s Theorem. However, I did not parameterize CaO at that time. I was pleased to find that the Herzberg and O’Hara2 method now agrees with the new CaO parameterization for the high CaO HSDP2 lavas, consistent with peridotite source melting at Mauna Kea. There is also internal consistency for other picritic occurrences such as Gorgona, and Baffin Island (Table 1, text). However, this consistency breaks down for most Hawaiian tholeiites because they are too low in CaO to 2 be peridotite-source melts (Figure 1b). The Herzberg2,14 models for the Hawaiian tholeiites are now wrong because our assumed peridotite source composition was wrong. A pyroxenite source is preferred. Phase Diagrams for Pyroxenite Sources Reviews of phase equilibria pertinent to this discussion are given by Milholland and Presnall15 for the system CaO-MgO-Al2O3-SiO2 and Kogiso et al.16 for natural pyroxenitic compositions. Addition of TiO2, Cr2O3, FeO, Na2O, K2O to the system CaOMgO-Al2O3-SiO2 can affect the phase diagrams in complex ways that have yet to undergo experimental calibration. Consequently, only compositions in natural complex systems comparable to potential Hawaiian source and primary magma compositions will be considered here. The pyroxene-garnet plane is a thermal divide that separates liquids on each side from being derived from each other either by partial melting or crystallization15-17. It is defined by pyroxene and garnet compositions that plot within the plane DiopsideEnstatite-Pyrope-Grossular (Figure S4). To visualize this as a divide, we will use the projection Diopside into the plane Olivine-Quartz-CATS (i.e., calcium Tschermak’s pyroxene) as recommended by Kogiso et al.16, using the projection code of O’Hara18; all pyroxene and garnet compositions project along the line Opx-CATS. All source compositions on the olivine-rich side of the thermal divide will form partial melt compositions that remain on the olivine-rich side of the divide. Similarly, all source compositions on the SiO2-rich side of the thermal divide will form partial melt compositions that remain on the SiO2-rich side of the divide (Figure S4). We are particularly interested in liquid compositions that are in equilibrium with orthopyroxene + clinopyroxene + garnet, and will use the notation [L+Opx+Cpx+Gt]. Experimental data19-23 on compositions close to those of the divide permit the liquidus crystallization fields to be mapped out at 3 GPa (Figure S4b). Data by Keshav et al.23 are at 2.5 GPa rather than 3.0 GPa, and provide a bound for liquids coexisting with Cpx+Gt. For the more olivine-rich equilibrium [L+Ol+Opx] at 3 GPa we use experimental data from Walter1 and its parameterization2. Likewise, data from Walter1 are used for the equilibrium [L+Ol+Opx+Cpx]. The cotectics [L+Ol+Opx+Cpx] and [L+Opx+Cpx+Gt] intersect at a pseudoinvariant point [L+Ol+Opx+Cpx+Gt] (Figure S4a,c). This is a wellknown peritectic reaction point defined by L+Opx=Ol+Cpx+Gt2,17. At lower temperatures, Opx disappears and liquids evolve along the cotectic [L+Ol+Cpx+Gt]. Data of Keshav et al.23 and Hirschmann et al.24 for [L+Cpx+Gt] at 2.5 GPa are used because they provide a bound on [L+Ol+Cpx+Gt] at 3.0 GPa. Although this equilibrium is not particularly important for most melt generation below Hawaii, it is considered here for purposes of completeness. And although the 2.5 GPa experimental liquids must be slightly deficient in MgO and enriched in Al2O3 compared to [L+Ol+Cpx+Gt] at 3.0 GPa, they provide a good visual sense of the projected distribution of this cotectic. To the Si-rich side of the pyroxene –garnet thermal divide, no experimental data exist on [L+Opx+Cpx+Gt] that are relevant to Hawaii. However, data of Pertermann and Hirschmann25 on a MORB-like eclogite provide important constraints. Shown in Figure S5 are their data for liquids for the equilibrium [L+Qz+Cpx+Gt], and its likely intersection with [L+Opx+Cpx+Gt]. This intersection defines a pseudoinvariant point involving [L+Qz+Opx+Cpx+Gt], and it is drawn as a eutectic. The Pertermann and 3 Hirschmann data are important because they indicate the cotectic for [L+Opx+Cpx+Gt] projects linearly on each side of the thermal divide as shown in Figure S5, and it is also likely to be orthogonal to the thermal divide. This [L+Opx+Cpx+Gt] cotectic is indicated as a broken line to emphasize it is an interpolation that requires testing by experiment. However, this linear behavior can be viewed with some confidence because it is very similar to low-pressure phase equilibrium studies. For example, at 1 atmosphere the cotectic [L+Forsterite +Anorthite +Augite] in the system CaO-MgO-Al2O3-SiO2 contains a thermal divide which separates liquids with low and high SiO2 contents26,27. This cotectic also projects orthogonal to and linearly on each side of its divide, as does the cotectic in more complex systems28; crystallization along it is responsible for much of the petrological variation in mid-ocean ridge basalts29,30. Experimental data at 4 GPa are few. Only the data of Walter1 and its parameterization2 exist, and these characterize the compositions of liquids for the olivinerich equilibria [L+Ol+Opx], [L+Ol+Cpx+Gt], and [L+Ol+Opx+Cpx+Gt]. These are shown in Figure S6. The effect of increasing pressure from 3 to 4 GPa is to expand the garnet and orthopyroxene liquidus crystallization fields at the expense of olivine, observations that have been described in considerable detail elsewhere2. Figure S7 is a summary of the essential phase diagram at 3 and 4 GPa. Liquid in [L+Opx+Cpx+Gt] within the garnet-pyroxene plane at 3 GPa is well-constrained, and has the composition 3a indicated in Figure S7 and Table S1. Liquid at the olivine-rich termination of this 3 GPa cotectic [L+Ol+Opx+Cpx+Gt] is also well-constrained, and has the composition 3b in Figure S7 and Table S1. At 4 GPa, liquid [L+Ol+Opx+Cpx+Gt] is known (i.e., 4b), but the composition of liquid for [L+Opx+Cpx+Gt] within the garnetpyroxene plane is not. A linear exptrapolation of the 4 GPa cotectic [L+Opx+Cpx+Gt] constrains the composition 4a in Table S1. Future experimental studies are required to calibrate these 4 GPa compositions. However, the linear behavior of [L+Opx+Cpx+Gt] at 3 GPa gives us some confidence in this extrapolation. Experimental data of Yaxley and Green31 at 3.5 GPa have not been plotted; however, they are in good agreement with bounds imposed by the cotectics in Figure S7. HSDP Whole Rock Fractionation Variations in whole rock SiO2, MgO, and CaO displayed in Figures 1 and 2 of the text are mostly the products of olivine phenocryst addition and subtraction. However, there is a small component of plagioclase and augite addition and subraction that is not obvious in these plots, and it compromises the model primary magma compositions that have been computed by projection. This is clearly evident in Figure S8, where the model pyroxenite primary magmas for whole rocks are compared with those of the glass analyses in a diagram that depicts liquids that crystallize olivine+plagioclase+diopside [L+Ol+Plag+Aug]30. Many whole rocks are coincident with glasses, but others scatter as outliers towards vectors defined by plagioclase and plagioclase+augite. The outliers were filtered out of the whole rock model pyroxenite melt compositions, and the remaining ones used in Figures 3 and 4 of the text. This is the most simple model-independent procedure for correcting for minor plagioclase + augite fractionation, and facilitates a direct comparison of model primary magma compositions derived from whole rocks and glasses. 4 Second-stage Pyroxenite Sources Direct partial melting an original basaltic crustal protolith yields partial melts in equilibrium with eclogite or quartz/coesite eclogite at 3.0-3.5 GPa25,31. These melts are too low in NiO, MgO, and high in SiO2 to match Hawaiian tholeiites25,31. They are also too enriched in Al2O3 to be in equilibrium with orthopyroxene (Figure S9). Likewise, the primitive Mauna Kea magmas in equilibrium with Fo90.5 olivine11 are too deficient in Al2O3 to be in equilibrium with a bimineralic eclogite source (Figure 2, text). How does the source develop orthopyroxene? Sobolev et al.32 proposed a second-stage melt-rock reaction process, and this is illustrated in Figure S9. At 3 GPa, quartz eclogite begins to melt at about 1315oC, and advanced melts can leave behind a residue of bimineralic eclogite25,31. Reaction of the SiO2-rich melts with the peridotite host31-33 is illustrated in Figure S9. Yaxley and Green31 reported normal peridotite and eclogite separated by an orthopyroxene-rich reaction layer of with minor Ol+Cpx+Gt. The compositions of the 2 pyroxenes and garnets at this Opx-rich layer are shown in Figure S9b. High degree melts of eclogitic crust react with peridotite to produce Cpx+Gt. Low degree melts of eclogitic crust react with peridotite to produce Opx+Cpx+Gt, the pyroxenite source for high SiO2 melts at Mauna Kea. Sobolev et al.32 report a second stage reaction produce of Cpx+Gt, but Opx is likely to be important. References 1. Walter, M.J. Melting of garnet peridotite and the origin of komatiite and depleted lithosphere. Journal of Petrology 39, 29-60 (1998). 2. Herzberg, C. & O’Hara, M.J. Plume-associated ultramafic magmas of Phanerozoic age. Journal of Petrology 43, 1857-1883 (2002). 3. Herzberg, C. Geodynamic information in peridotite petrology. Journal of Petrology, 45, 2507-2530 (2004). 4. Baker, M.B. & Stolper, E.M. Determining the composition of high-pressure mantle melts using diamond aggregates. Geochimica et Cosmochimica Acta 58, 2811-2827 (1994). 5. Baker, M.B., Hirschmann, M.M., Ghiorso, M.S. & Stolper, E.M. Compositions of near-solidus peridotite melts from experiments and thermodyamic calculations. Nature 375, 308-310 (1995). 6. Hirschmann, M.M., Baker, M.B. & Stolper, E.M. The effect of alkalis on the silica content of mantle-derived melts. Geochimica et Cosmochimica Acta 62, 883-902 (1998). 7. Robinson, J.A.C., Wood, B.J. & Blundy, J.D. The beginning of melting of fertile and depleted peridotite at 1.5 GPa. Earth and Planetary Science Letters 155, 97-111 (1998). 8. Hirose, K. & Kushiro, I. Partial melting of dry peridotites at high pressures: determination of compositions of melts segregated from peridotite using aggregates of diamond. Earth and Planetary Science Letters 114, 477-489 (1993). 9. Kushiro, I. in Earth Processes: Reading the Isotopic Code (eds Basu, A. & Hart, S.) 109-122 (Vol 95, Geophysical Monograph Series, AGU, Washington, DC 1996). 10. Humayun, M., Qin, L. & Norman, M.D. Geochemical evidence for excess iron in the mantle beneath Hawaii, Nature 306, 91-94 (2004). 5 11. Stolper, E., Sherman, S., Garcia, M., Baker, M. & Seaman, C. Glass in the submarine section of the HSDP2 drill core, Hilo, Hawaii. Geochemistry, Geophysics, Geosystems 5, doi:10,1029/2003GC000553 (2004). 12. Longhi, J. Some phase equilibrium systematics of lherzolite melting: I. Geochemistry, Geophysics, Geosystems 3(3), doi:10,1029/2001GC000204 (2002). 13. Salters, V.J.M., Longhi, J.E. & Bizimis, M. Near mantle solidus trace element partitioning at pressures up to 3.4 GPa. Geochemistry, Geophysics, Geosystems 3(7) doi:10,1029/2001GC000148 (2002). 14. Feigenson, M.D., Bolge, L.L., Carr, M.J. and Herzberg, C.T. REE Inverse modeling of HSDP2 basalts: Evidence for multiple sources in the Hawaiian plume. Geochemistry, Geophysics, Geosystems 4(2), doi:10.1029/2001GC000271 (2003).. 15. Milholland, C. S. & Presnall, D. C. Liquidus phase relations in the CaO-MgO-Al2O3SiO2 system at 3.0 GPa: The aluminous pyroxene thermal divide and high pressure fractionation of picritic and komatiitic magmas, Journal of Petrology 39, 3-27 (1998). 16. Kogiso, T. Hirschmann, M.M. & Pertermann M. High-pressure partial melting of mafic lithologies in the mantle. Journal of Petrology 45, 2407-2422 (2004). 17. O’Hara, M.J. & Yoder, H.S. Jr. Formation and fractionation of basic magmas at high pressures. Scottish Journal of Geology 3, 67-117 (1967). 18. O’Hara, M.J. The bearing of phase equilibria studies in synthetic and natural systems on the origin of basic and ultrabasic rocks. Earth Science Reviews 4, 69-133 (1968a). 19. Ito, K. & Kennedy, G.C. Melting and phase relations in the plane tholeiite-lherzolitenepheline basanite to 40 kilobars and geological implications. Contributions to Mineralogy and Petrology 19, 177-211 (1968). 20. Ito, K. & Kennedy, G.C. The composition of liquids formed by partial melting of eclogite at high temperatures and pressures. Journal of Geology 82, 383-392 (1974). 21. Eggins, S.M. Petrogenesis of Hawaiian tholeiites: 1, phase equilibria constraints. Contributions to Mineralogy and Petrology 110, 387-397 (1992). 22. Maaloe, S. The PT-phase relations of an MgO-rich Hawaiian tholeiite: the compositions of primary Hawaiian tholeiites. Contributions to Mineralogy and Petrology 148, 236-246 (2004). 23. Keshav, S., Gudfinnsson, G.H., Sen, G. & Fei, Y. High-pressure melting experiments on garnet clinopyroxenite and the alkalic to tholeiitic transition in ocean-island basalts. Earth and Planetary Science Letters 223, 365-379 (2004). 24. Hirschmann, M.M., Kogiso, T., Baker, M.B. & Stolper, E.M. Alkalic magmas generated by partial melting of garnet pyroxenite. Geology 31, 481-484 (2003). 25. Pertermann, M. & Hirschmann, M.M. Anhydrous partial melting experiments on MORB-like eclogite: phase relations, phase compositions and mineral-melt partitioning of major elements at 2-3 GPa. Journal of Petrology 44, 2173-2201 (2003). 26. Libourel, G., Boivin, P. & Biggar, G.M. The univariant curve liquid = forsterite + anorthite + diopside in the system CMAS at 1 bar: solid solutions and melt structure. Contributions to Mineralogy and Petrology 102, 406-421 (1989). 27. Longhi, J. Liquidus equilibria and solid solution in the system CaAl2Si2O8-Mg2SiO4CaSiO3-SiO2 at low pressure. American Journal of Science 287, 265-331 (1987). 6 28. Sack, R.O., Walker, D. & Carmichael, I.S.E. Experimental petrology of alkalic lavas: constraints on cotectics of multiple saturation in natural basic liquids. Contributions to Mineralogy and Petrology 96, 1-23 (1987). 29. O’Hara, M.J. Are ocean floor basalts primary magmas? Nature 220, 683-686(1968b). 30. Herzberg, C. Partial crystallization of mid-ocean ridge basalts in the crust and mantle, Journal of Petrology, 45, 2389-2405 (2004). 31. Yaxley, G.M. & Green, D.H. Reactions between eclogite and peridotite: mantle refertilisation by subduction of oceanic crust. Schweiz. Mineral. Petrogr. Mitt., 78, 243-255 (1998). 32. Sobolev, A.V., Hofmann, A.W., Sobolev, S.V. & Nikogosian, I.K. An olivine-free mantle source of Hawaiian shield basalts, Nature, 434, 590-597 (2005). 33. Takahashi, E. & Nakajima, K. in Hawaiian Volcanoes: Deep Underwater Perspectives (eds Takahashi, E., Lipman, P.W., Garcian, M.O., Naka, J. & Aramaki, S.) 403-418 (Vol 128, Geophysical Monograph Series, AGU, Washington DC, 2002). 7