ME 210: Material Science Fall 2015 Assignment #4 Packing
advertisement

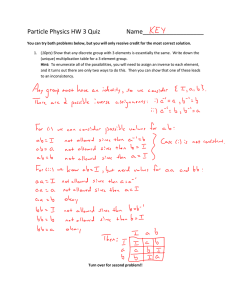
ME 210: Material Science Fall 2015 Assignment #4 Packing Densities and Coordination Name______________________________ Banner ID____________________ Date___________ 1. Calculate the radius of an iridium atom, given that Ir has an FCC crystal structure, a density of 22.4 g/cm3, and an atomic weight of 192.2 g/mol. (10pts) a. 0.136 nm b. 0.201 nm c. 0.151 nm d. 0.124 nm 2. From the data in Table 3.4 in your text, compute the theoretical density of CaF2, which has the fluorite structure. (10pts) a. 4.01 g/cm^3 b. 3.33 g/cm^3 c. 2.97 g/cm^3 d. 3.78 g/cm^3 3. How many ions are in the unit cell of CaO? (5pts) a. 2 b. 4 c. 6 d. 8 4. What is the ionic packing factor, in %, for CaO?____(list three significant figures) a. 59.3% b. 61.3% c. 56.7% d. 54.7%