Disease name
advertisement

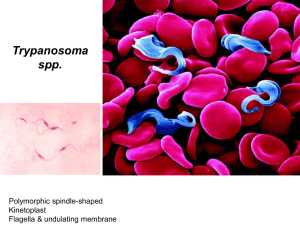
Activities in 2009 Surra (Trypanosoma evansi) Dr Noboru Inoue National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, 2-11, Inada, Obihiro, Hokkaido 080-8555, Japan Tel.: +81 155 495647, Fax: +81 155 495643 E-mail: ircpmi@obihiro.ac.jp, Website: http://www.obihiro.ac.jp/~tryp/eng_index.html Summary of general activities related to the disease 1. 2. Test(s) in use/or available for the specified disease at your laboratory Test For Specificity Total IFAT Antibody Salivarian trypanosome 0 ELISA Trypanosome cell lysate Antibody Salivarian trypanosome 203 ELISA Recombinant P0 Antibody Salivarian trypanosome 0 PCR/T. evansi DNA Trypanosoma evansi 30 PCR/Trypanozoon DNA Trypanozoon 671 PCR/SRA DNA T. b. rhodesiense 671 PCR/TgsGP DNA T. b. gambiense 203 PCR/T. congolense All subtypes DNA T. congolense All subtypes 671 LAMP/Trypanozoon DNA Trypanozoon 671 LAMP/T. congolense DNA T. congolense 438 Microscopy Parasite Trypanosoma 491 Production and distribution of diagnostic reagents A. Trypanosoma brucei and Trypanosoma congolense procyclic form crude antigen for use in antibody detection ELISA. B. Recombinant T. congolense ribosomal P0 protein (apporx. 5 mg) for antigen detection ELISA was produced. C. LAMP Reagents (2 kits, 192 reactions per kit), Trypanozoon specific LAMP primer, and PCR primer sets (TBR, RoTat1.2, all subtypes of T. congolense) were sent to South Africa (Dr. Oriel Thekisoe) D. LAMP primer sets for detection of Trypanozoon (Target gene PFR), applox. 5,000 tests Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 1 Surra (Trypanosoma evansi) E. LAMP primer set for detection of T. congolense (approx. 5,000 tests) F. None of those were supplied to OIE members because of no requests. Activities specifically related to the mandate of OIE Reference Laboratories 3. International harmonisation and standardisation of methods for diagnostic testing or the production and testing of vaccines Continued from 2008, in collaboration with the Foundation for Innovative New Diagnostics (FIND, Switzerland) and EIKEN CHEMICAL CO LTD., development of Trypanozoon specific LAMP kit was carried out. This will be completed within a year 2010. 4. Preparation and supply of international reference standards for diagnostic tests or vaccines We can provide the following trypanosome DNA and crude antigens as a standards for diagnostic tests. However, we cannot provide live parasites due to legal restrictions in Japan. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 23 5. Species Trypanosoma brucei brucei Trypanosoma brucei brucei Trypanosoma brucei brucei Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma brucei gambiense Trypanosoma evansi Trypanosoma evansi Trypanosoma evansi Trypanosoma evansi Trypanosoma evansi Trypanosoma evansi Trypanosoma evansi Trypanosoma congolense Trypanosoma congolense Trypanosoma congolense Strain GUTat3.1 221 427 IL1922 IL3248 IL3250 IL3253 IL3254 IL3301 IL3707 Welcome IL2343 IL1695 IL1934 IL3354 IL3382 IL3960 IL3962 Tansui IL1180 IL3000 IL3338 Research and development of new procedures for diagnosis and control Trypanozoon specific LAMP kit for human African trypanosomosis will be developed within a year (2010) in collaboration with FIND and EIKEN CHEMICAL CO LTD. Validation based on OIE protocol of the kit will be carried out after completion of the development. Financed by Japan Society for the Promotion of Science (JSPS): Development of Trypanozoon specific immunochromatographic test (ICT) will start in 2010. Financed by JSPS: Epidemiological study on animal trypaosomoses were carried out in Northern Province in Zambia and in Northern region of Tanzania, and Southern Uganda. 2 Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 Surra (Trypanosoma evansi) 6. Collection, analysis and dissemination of epizootiological data relevant to international disease control (1) Molecular parasitology 1) Li, S. Q., Reid, S. A., Fung, M. C., Inoue, N. and Lun, Z. R. (2009) Analysis of gene expression profiles in the liver and spleen of mice infected with Trypanosoma evansi by using a cDNA microarray. Parasitology Research 104 (2) 385-397. 2) Mekata, H., Konnai, S., Witola, W. H., Inoue, N., Onuma, M. and Ohashi, K. (2009) Molecular detection of trypanosomes in cattle in South America and genetic diversity of Trypanosoma evansi based on expression-siteassociated gene 6. Infection, Genetics and Evolution 9 (6) 1301-1305. In South American countries, trypanosomiasis as a result of Trypanosoma evansi and Trypanosoma vivax infections causes significant economic losses in livestock. The objectives of this study were to characterize the epidemiology of bovine trypanosomiasis in South America and to draw a comparison between South American and Asian T. evansi isolates based on the polymorphisms in their transferrin receptor encoding gene 6. We assessed the prevalence rates of T. evansi and T. vivax infections in cattle in different regions of Peru and Bolivia using the polymerase chain reaction (PCR) and found that, in Lima and Pucallpa in the Republic of Peru, T. evansi infection rates were 5.8% (6/104) and 2.5% (5/195), respectively, while in Santa Cruz, Republic of Bolivia, the infection rate for T. evansi was 11.5% (59/510). The prevalence rates of T. vivax in Lima and Santa Cruz were 3.8% (4/104) and 0.9% (5/510), respectively. In T. evansi, uptake of host transferrin is mediated by a receptor derived from the two expression site-associated genes 6 and 7 (ESAG6 and ESAG7). We previously showed that the ESAG6 depicts genetic diversity among different isolates of T. evansi in Asia. In this study, we cloned and sequenced the ESAG6 genes from T. evansi isolates from South America, and found, in addition to some of the previously observed variants, 20 novel variants of ESAG6 genes which could be categorized into three new clades among the various isolates. To conclude, the results obtained in this study suggest that T. evansi isolates from South America are more diverse than the Asian isolates. (2) Epidemiology and development of diagnostics 1) Konnai, S., Mekata, H., Mingala, C. N., Abes, N. S., Gutierrez, C. A., Herrera, J. R. V., Dargantes, A. P., Witola, W. H., Cruz, L. C., Inoue, N., Onuma, M. and Ohashi, K. (2009) Development and application of quantitative real-time PCR for the diagnosis of Surra in water buffaloes. Infection, Genetics and Evolution 9 (4) 449-452. Trypanosoma evansi (T. evansi) causes the disease called Surra in domestic animals, which is of great economic importance in South Asian countries. In order to improve the diagnosis of Surra, we endeavored to develop a realtime PCR assay for the detection and quantification of parasites in water buffaloes using specific primers for the T. evansi antigen type (RoTat) 1.2 Variable Surface Glycoprotein (VSG) gene, which is a known diverse DNA region in trypanosomes. The quantitative detection limit of the assay was 10 2 trypanosomes per mL of blood, and the identity of the amplicon was confirmed in all assays by melting curve analysis. To evaluate the clinical applicability of this procedure, detection and estimation of parasitemia in blood samples obtained from water buffaloes and horses were conducted. T. evansi was detected in 17/607 (2.8%) blood samples, with parasitemia levels ranging from >10 to 107 parasites per mL of blood. Interestingly, out of the 17 PCR positive animals, 3 had previously received trypanocidal treatment and 1 had abortion history. These data indicate that real-time PCR for the estimation of putative parasitemia levels is a quantitatively and objectively applicable technique for clinical diagnosis of Surra, and could help to understand disease stage and risk of transmission of T. evansi. 2) Jing, Z., Magona, J. W., Sakurai, T., Thekisoe, O. M. M., Otim, C. P., Sugimoto, C. and Inoue, N. (2009) A field study to estimate the prevalence of bovine African trypanosomosis in Butaleja district, Uganda. The Journal of Veterinary Medical Science 71 (4) 525-527. Prevalence of bovine trypanosomosis was determined from a total of 203 blood samples collected from Butaleja district, eastern Uganda. All samples were examined by microhematocrit centrifuge test (MHC), PCR and ELISA. ELISA was performed in accordance with the OIE standard procedures using Trypanosoma brucei gambiense procyclic form crude antigens. PCR were utilized to identify the species and the subspecies of trypanosome. The overall prevalence of bovine African trypanosomosis was 8.9% by MHC, and 45.3% by the ELISA. Since substantial number (12 out of 18) of MHC positive samples were negative in the PCR tests, we could not conclude the most epidemic trypanosome species in the studied area. Nevertheless, the PCR results suggests that the most Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 3 Surra (Trypanosoma evansi) prevalent trypanosome was T. b. brucei (31/203), followed by T. congolense (6/203). In addition, only a few (3/203) mixed infections of T. b. brucei and T. congolense was detected by the PCR. Results obtained from this study indicates that bovine trypanosomosis is endemic in Butaleja district, Uganda. 3) Thekisoe, O. M. M., Bazie, R. S., Coronel-Servian, A. M., Sugimoto, C., Kawazu, S. and Inoue, N. (2009) Stability of loop-mediated isothermal amplification (LAMP) reagents and its amplification efficiency on crude trypanosome DNA templates. The Journal of Veterinary Medical Science 71 (4) 471-475. This study evaluated the stability of LAMP reagents when stored at 25 degrees C and 37 degrees C, and also assessed its detection efficiency on different DNA template preparations. Accordingly, LAMP using reagents stored at 25 degrees C and 37 degrees C amplified DNA of in vitro cultured T. b. brucei (GUTat 3.1) from day 1 to day 15 of reagent storage. There were no significant differences (P>0.05) in detection sensitivity of LAMP among the reagents stored at 25 degrees C, 37 degrees C and -20 degrees C (recommended storage temperature). LAMP using the reagents stored at above-mentioned temperatures amplified serially diluted DNAs (genomic DNA extracted by phenol-chloroform method, FTA card and hemolysed blood) of T. b. gambiense (IL2343) with high sensitivity. Reactions were conducted on the reagents stored from 1 day to 30 days. LAMP detection sensitivity was poor when fresh blood as DNA template was added directly into reactive solution. Results of this study demonstrated that LAMP has the potential to be used in field conditions for diagnosis of trypanosome infections without being affected by ambient temperatures of tropical and sub-tropical countries where trypanosomosis is endemic. 7. Provision of consultant expertise to OIE or to OIE Members Assistance to Dr. Louis Touratier, Secretary-general of the OIE ad hoc group on non-tsetse transmitted animal trypanosomoses (T. evansi and T. equiperdum). The Ad hoc Group meeting on Diagnostic Tests for Trypanosomoses was held from 30 March to 1 April 2009 at the OIE Headquarters, Paris. We have done revision of the diagnostic manual for Surra. 8. Provision of scientific and technical training to personnel from other OIE Members Supported by Japan International Cooperation Agency (JICA): Trainees from China and Zimbabwe were trained for improvement of their basic molecular diagnostic techniques for 10 months. Financed by Japan Society for the Promotion of Science (JSPS), and National Research Foundation (NRF) in South Africa: Tanzanian, and South African scientists were trained for improvement of their basic molecular parasitology skills for 2 weeks. 9. Provision of diagnostic testing facilities to other OIE Members None 10. Organisation of international scientific meetings on behalf of OIE or other international bodies None 11. Participation in international scientific collaborative studies Financed by JSPS: Study of the animal trypanosomoses in Zambia and Tanzania. Molecular epidemiological survey of animal trypanosomoses was carried out. Counterpart: Dr. Boniface Namangala, University of Zambia, and Prof. Chihiro Sugimoto, Hokkaido University. Total blood DNA from 402 cattle and 36 goats were subjected to PCR or LAMP tests for Trypanozoon, T. b. rhodesiense, T. congolense and T. vivax. As a results, 4 (Trypanozoon), 23 (T. congolense), 1 (T. b. rhodesiense) and 6 (T. vivax) positive animals were detected either PCR or LAMP tests. 4 Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 Surra (Trypanosoma evansi) Financed by JSPS: Study of the animal trypanosomoses in Uganda. Molecular epidemiological survey of animal trypanosomoses was carried out. Counterpart: Dr. Magona, J.W., NaLIRRI, Uganda, and Prof. Chihiro Sugimoto, Hokkaido University. Details are shown above in Chapter 6. 12. Publication and dissemination of information relevant to the work of OIE (including list of scientific publications, internet publishing activities, presentations at international conferences) Scientific publications in peer-reviewed journals 1) Helm, J. R., Hertz-Fowler, C., Aslett, M., Berriman, M., Sanders, M., Quail, M. A., Soares, M. B., Bonaldo, M. F., Sakurai, T., Inoue, N. and Donelson, J. E. (2009) Analysis of expressed sequence tags from the four main developmental stages of Trypanosoma congolense. Molecular and Biochemical Parasitology 168 (1) 34-42. 2) Mekata, H., Konnai, S., Witola, W. H., Inoue, N., Onuma, M. and Ohashi, K. (2009) Molecular detection of trypanosomes in cattle in South America and genetic diversity of Trypanosoma evansi based on expression-siteassociated gene 6. Infection, Genetics and Evolution 9 (6) 1301-1305. 3) Konnai, S., Mekata, H., Mingala, C. N., Abes, N. S., Gutierrez, C. A., Herrera, J. R. V., Dargantes, A. P., Witola, W. H., Cruz, L. C., Inoue, N., Onuma, M. and Ohashi, K. (2009) Development and application of quantitative real-time PCR for the diagnosis of Surra in water buffaloes. Infection, Genetics and Evolution 9 (4) 449-452. 4) Li, S. Q., Reid, S. A., Fung, M. C., Inoue, N. and Lun, Z. R. (2009) Analysis of gene expression profiles in the liver and spleen of mice infected with Trypanosoma evansi by using a cDNA microarray. Parasitology Research 104 (2) 385-397. 5) Jing, Z., Magona, J. W., Sakurai, T., Thekisoe, O. M. M., Otim, C. P., Sugimoto, C. and Inoue, N. (2009) A field study to estimate the prevalence of bovine African trypanosomosis in Butaleja district, Uganda. The Journal of Veterinary Medical Science 71 (4) 525-527. 6) Sakurai, T., Tanaka, M., Kawazu, S. and Inoue, N. (2009) Establishment of an in vitro transgene expression system in epimastigotes of Trypanosoma congolense. Parasitology International 58 (1) 110-113. 7) Thekisoe, O. M. M., Bazie, R. S., Coronel-Servian, A. M., Sugimoto, C., Kawazu, S. and Inoue, N. (2009) Stability of loop-mediated isothermal amplification (LAMP) reagents and its amplification efficiency on crude trypanosome DNA templates. The Journal of Veterinary Medical Science 71 (4) 471-475. 13. Inscription of diagnostic kits on the OIE Register i) Did you participate in expert panels for the validation of candidate kits for inscription on the OIE Register? If yes, for which kits? None ii) Did you submit to the OIE candidate kits for inscription on the OIE Register? If yes, for which kits? None _______________ Annual reports of OIE Reference Laboratories and Collaborating Centres, 2009 5