Electricity and Magnetism Technical Committees of the RMOs
advertisement

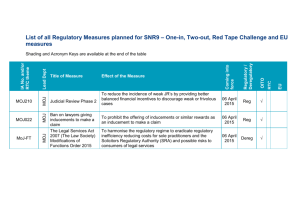
Meeting of the Chairpersons of the Electricity and Magnetism Technical Committees of the RMOs BIPM, 3 November 2003, h 9:00 – 12:00 RMOEM 03-09 p. 1/2 END OF TRANSITION PERIOD OF CIPM MRA – REVIEW OF PUBLISHED CMCs During the transition period, many NMIs1 are in the process of participating in comparison programs and establishing their quality systems. Thus, CMCs being published during this period are based on fulfilment, as far as possible, of the criteria given in the Document JCRB-8/13(1b) “Criteria for acceptance of data for Appendix C”. For convenience these criteria are copied below: 1. Results of key and supplementary comparisons.2 2. Documented results of past CCs, RMO or other comparisons (including bilateral comparisons). 3. Knowledge of technical activities by other NMIs. 4. Active participation in RMO projects. 5. Appropriate measurement procedures and equipment. 6. Scientific and technical qualifications of staff. 7. Other available knowledge and experience. 8. Quality system existing or in preparation, brief description. 9. Any peer assessment, third party accreditation or self declaration, including the name of the accreditation body; membership of a multilateral agreement/arrangement; scope of accreditation; names of peer reviewers. Following the end of the transition period of the CIPM MRA, i.e., after 31 December 2003 (see Document JCRB-8/13(1)), CMCs submitted for publication on the BIPM MRA website will be required to have, as their basis, evidence of fulfilment of the criteria listed above under points 1-9, with special emphasis on the following points: A. With respect to Criterion 1, it is the on-going responsibility of the Working Group on CMCs within each Consultative Committee to monitor the results of key and supplementary comparisons and provide a written report to the JCRB in the case that these results affect published CMCs. The relevant RMO representative to the JCRB transmits this report as appropriate within its RMO. It is the responsibility of the NMI providing the CMCs to notify the KCDB Coordinator in order to undertake appropriate action. Such action may involve increasing the uncertainties of CMCs or withdrawing CMCs. The relevant RMO will keep the JCRB informed of the status of such CMCs. In this document all references to “NMIs” also include “designated institutes”. NMIs that have not yet taken part in key or supplementary comparisons are required as a minimum to have traceability of their national standards established through calibration by an NMI that has established its degree of equivalence through participation in the key comparison programs; they must also have participated in some bilateral comparisons in addition to meeting the other criteria listed here. 1 2 Meeting of the Chairpersons of the Electricity and Magnetism Technical Committees of the RMOs BIPM, 3 November 2003, h 9:00 – 12:00 RMOEM 03-09 p. 2/2 B. With respect to Criterion 8, after the end of the transition period, CMCs published in the KCDB must be supported by a Quality System. The JCRB, at its 11th Meeting, decided that it is the responsibility of the RMO representative to the JCRB to provide the JCRB Chairman by 5th April 2004 with a comprehensive report on the status of the Quality System of each member NMI, following the “JCRB Guidelines for the monitoring and reporting of the operation of Quality Systems by RMOS” [Doc –JCRB-10/8(1c)]. It is in consequence the responsibility of each NMI to provide its RMO with the appropriate information regarding its Quality System to enable the RMO to meet the deadline of 5th April 2004. In its report to the JCRB Chairman, the RMO shall state the range of CMCs covered by the Quality System and, for those CMCs that are not adequately covered, shall provide a reasonable timeframe by which these will also be covered. For CMCs that are not adequately covered by a Quality System, in the absence of notification by the RMO to the JCRB Chairman of a timeframe and JCRB approval of this timeframe, or in the event that the approved timeframe is not kept, the CMCs will be withdrawn from the KCDB until such time as the Quality System criterion is fulfilled. C. In cases where an NMI has used “provisional” evidence (i.e., not based on the results of key or supplementary comparisons) to support CMCs during the transition period, it is the responsibility of the NMI, through its RMO, to provide the JCRB with more substantive evidence (i.e., based on the results of key or supplementary comparisons) to support these CMCs as soon as it becomes available. It is the responsibility of each RMO to monitor these cases. By 31 December 2003, RMOs must inform the JCRB Chairman if they require additional evidence after the end of the transition period to support any CMCs of another RMO that have already been published. The JCRB Chairman will then notify the originating RMO of these requests. In these cases, the originating RMO must inform the JCRB Chairman by 5th April 2004 what action it proposes to take to address the issues raised. Alternatively, if an NMI wishes to withdraw previously published CMCs at the end of the transition period, the RMO-JCRB Representative must inform the JCRB Chairman by the end of the transition period. All actions relating to published CMCs that are to be amended in any way following the end of the transition period should be tabled at the 2nd meeting of the JCRB in 2004.