Gas – Solid Reactions
advertisement
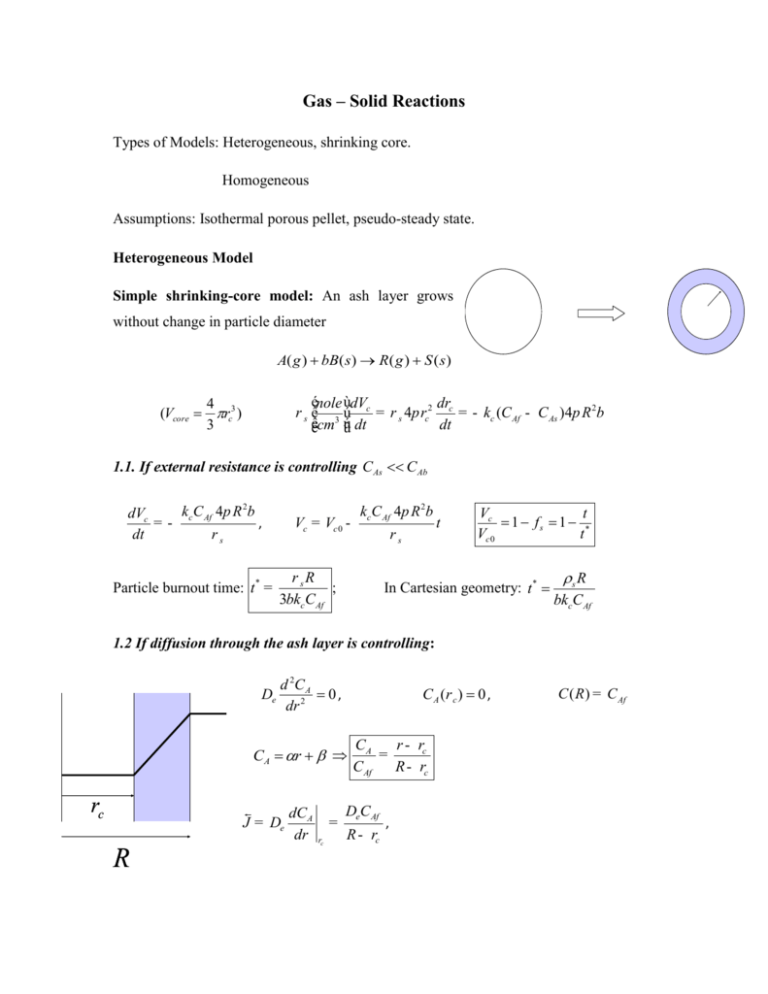
Gas – Solid Reactions Types of Models: Heterogeneous, shrinking core. Homogeneous Assumptions: Isothermal porous pellet, pseudo-steady state. Heterogeneous Model Simple shrinking-core model: An ash layer grows without change in particle diameter A( g ) bB( s ) R( g ) S ( s ) énole ùdV dr r s ê 3 ú c = r s 4p rc2 c = - kc (C Af - C As )4p R 2b êëcm úû dt dt 4 (Vcore rc3 ) 3 1.1. If external resistance is controlling CAs C Ab kc C Af 4p R 2b dVc =, dt rs Particle burnout time: t * = Vc = Vc 0 - kc C Af 4p R 2b rs r sR ; 3bkcC Af t Vc t 1 fs 1 * Vc 0 t In Cartesian geometry: t * s R bkcCAf 1.2 If diffusion through the ash layer is controlling: De d 2C A 0, dr 2 C A (r c ) 0 , CA r rc J = De R dC A dr = rc r - rc CA = CAf R - rc DeC Af R - rc , C ( R ) = C Af DeC Af dVc dr = Ar s c = - A b dt dt R - rc rs R- rc ò ( R - rc )d ( R - rc ) = R- rc = 0 1 ( R - rc )drc = ( R - rc )2 DeC Af b = t 2 rs 2 DeC Af b t 2 s R r t 2 1 c * , R t - DeC Af b rs dt 1 * 1.3 If an ash layer doesn’t grow, the particle diameter decreases. rs dVc dr = r s 4p rc2 c = - kc (C Af - C As )4p rc2b dt dt To a first order approximate kc is assumed to be independent on rc, and C As C Af : kc C Af b drc , =dt rs 3 t rc 1 f b 1 * R t rc t 1 * , R t 3 2. The general heterogeneous model: spherical pellet with both external and internal resistances; fast reaction occurs at r=rc at a rate of k’CAC (k’=[cm/s]) Shell balance: 2C A 0, C A C AC De dC A dr kc C Af C A , R De dC A dr k ' C AC rc De 1 1 1 k ' rc rc r 1 1 B C Af De 1 De 1 rc r 1 1 k ' rc rc kc R R To find rc(t) rewrite the solid phase balance (here Cs0=s denote molar solid concentration): dC A d 4 rc 3 Cso bDe dt 3 dr 4 rc 2 rc (*) Which after substitution of Eqn. (*) yields: r bC Af 1 1 1 rc 2 c dt dr c De k ' De k g R R Cso After integration from R to rc, the burning time is (c=rc/R): bCAf Cso t R 1 R R2 R 3 1 1 c2 1 c c 3 kc De 2 De k' Burnout time corresponds to c=0 and it varies like R for film-controlled or kineticscontrolled process and like R2 for pore-diffusion control We can find a relation of time and conversion (x=1-c3). When film-diffusion controls: t Cso R x 3bkcCAf while when pore-diffusion controls t Cso R 2 23 1 3 1 x 2 1 x bDeC Af 3. Nonisothermal pellet- Heterogeneous Model The rate of heat generation and heat removal, assuming the particle to be isothermal, are h TC T f R 2 H k ' C AC rc2 H C Af De rc 2 De 1 k ' rc 1 De 1 1 rc kc R R rc2 After using Eqn. (*) for CAC. The heat generation curve is temperature dependent [k’(T)] but also time-dependent (as rc varies with t)