First author, Year, Country [ref]
advertisement
![First author, Year, Country [ref]](http://s3.studylib.net/store/data/007545193_2-f76b71deabed5ffcefb465c1f9187c6f-768x994.png)
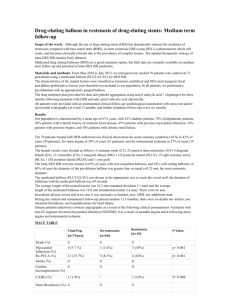
First author, Year, Country [ref] Study design, duration of FU Volzke, 2000, Germany [27] cohort 6 months Yoshida, 1999, Japan [28] Cohort description [No of patients (M/F), Ethnicity, Mean Age (SD), inclusion criteria] Outcome assessed (diagnostic criteria)* [Classification of outcome] Intervention Gene-gene interaction assessed? (gene) Quality score Main Findings [Calculated OR (95% Confidence Interval) or presentation of results when data where not available for OR calculation] Balloon Angioplasty PTCA-balloon No 35 Data synthesized in the metaanalysis Restenosis (2) [surrogate] PTCA-balloon No 30 Data synthesized in the metaanalysis Restenosis (1) [surrogate] cohort 5.21 (3.9) years cohort 6 months 511 (388/123), Eur Whites, 60.6 (8.6), CAD patients undergoing elective PTCA of a previously untreated native coronary artery 123 (nr), East Asians, 58.2 (10.2), MI patients undergoing PTCA discharged from hospital at the start of FU 69 (57/12), Eur Whites, 58 (9.9), UA patients undergoing PTCA Restenosis (3) [surrogate] PTCA-balloon No 27 Data synthesized in the metaanalysis Kamitani, 1995, Japan [30] Samani, 1995, UK [31] cohort 6 months 103 (103/00), East Asians, 52 (1), Pimary PTCA for MI patients Restenosis (1) [surrogate] PTCA-balloon No 27 Data synthesized in the metaanalysis cohort 4 months Restenosis (1) [surrogate] PTCA-balloon No 34 Data synthesized in the metaanalysis van Bockxmeer, 1995, Australia [32] Tsukada, 1997, Japan [33] cohort 6 months 233 (194/39), nr, 56 (1), single-vessel PTCA in the Subcutaneous Heparin and Angioplasty Restenosis Prevention (SHARP) study 207 (170/37), Eur Whites, 57 (9), CAD patients undergoing elective PTCA Restenosis (1) [surrogate] PTCA-balloon Yes (APOE) 33 Data synthesized in the metaanalysis cohort 3 months 96 (nr), East Asians, 60 (1.0), CAD patients undergoing elective PTCA Restenosis (3) [surrogate] PTCA-balloon No 28 Data synthesized in the metaanalysis Beohar, 1995, USA [34] cohort 3 months 89 (nr), Am Whites, 63.9 (10), CAD patients undergoing elective PTCA Restenosis (3) [surrogate] PTCA-balloon No 24 Data synthesized in the metaanalysis Zee, 2001, Spain [35] cohort 6 months 342 (305/37), Eur Whites, 58.9 (9.6), CAD patients undergoing PTCA Restenosis (1) [surrogate] PTCA-balloon No 37 Data synthesized in the metaanalysis Ohishi, 1993, Japan [36] cohort 6 months 82 (nr), East Asians, nr, MI patients undergoing primary PTCA Restenosis (1) [surrogate] PTCA-balloon No 23 Data synthesized in the metaanalysis Kasi, 1996, Spain [29] Hertwig, 2002, Germany [37] cohort 4-6 months 145 (128/17), Eur Whites, 59.2 (8.4), CAD patients undergoing repeat PTCA for restenotic lesions at FU angiography 52 (47/5), Eur Whites, nr, CAD patients undergoing PTCA Recurrent Restenosis (1) [surrogate] endothelial dysfunction (4) [surrogate] PTCA-balloon No 33 Mulder, 2003, Netherlands [38] crosssectional (PREFACE) Wijpkema, 2006, Netherlands [39] Gomma, 2002, UK [40] cohort 9 months 2888 (2050/838), Eur Whites, 62 (11), CAD patients undergoing elective PTCA Restenosis (5) [clinical] cohort 6 months 205 (155/50), Eur Whites, 59.4 (9.9), CAD patients undergoing PTCA Restenosis (3) [surrogate] PTCA-STENT Ruy, 2002, Korea [41] cohort 6 months 238 (178/60), East Asians, 59.5 (9.9), CAD patients undergoing PTCA Restenosis (3) [surrogate] Ribichini, 2004, Italy [42] Taniguchi, 2001, Japan [43] Koch, 2000, Germany [44] cohort 6 months 897 (160/737), Eur Whites, 61 (10), CAD patients undergoing PTCA cohort 6 months Allele contrast OR=1.24 (0.8-2.0) Domin model OR=1.79 (0.8-4.2) Recess model OR=0.90 (0.4-2.1) an ameliorating effect of pravastatin in patients with the DD genotype was found PTCA-balloon + Pravastatin (40mg) Angioplasty with stent deployment PTCA-STENT No 23 Yes (AGT, AGT1R, AGT2R, HMOX1) No 35 Data synthesized in the metaanalysis 27 Data synthesized in the metaanalysis PTCA-STENT Yes (CYP11B2 , AGT) 32 Restenosis (3) [surrogate] PTCA-STENT No 33 Data synthesized in the metaanalysis No significant gene-gene interaction was observed Data synthesized in the metaanalysis 67 (50/17), East Asians, 65.2 (9.7), CAD patients undergoing PTCA Restenosis (1) [surrogate] PTCA-STENT No 23 Data synthesized in the metaanalysis cohort 1 year 1850 (1458/392), Eur Whites, 62.9 (10), CAD patients undergoing PTCA Restenosis (3) [surrogate] PTCA-STENT No 40 Data synthesized in the metaanalysis Gurlek, 2000, Turkey [45] cohort 6 months 132 (112/20), Turks, 53 (9), CAD patients undergoing PTCA Restenosis (3) [surrogate] PTCA-STENT No 29 Data synthesized in the metaanalysis Hamon, 1998, France [46] cohort 6 months 271 (229/42), Eur Whites, 60 (10), CAD patients undergoing PTCA Restenosis (3) [surrogate] PTCA-STENT Yes (AGT1R) 34 Hamon, 1996 France [47] cohort nr 291 (nr), Eur Whites, nr, CAD patients undergoing PTCA Total occlusion [surrogate] PTCA-STENT No 20 Data synthesized in the metaanalysis No significant gene-gene interaction was observed Allele contrast OR=1.71 (0.8-3.6) Domin model OR=0.89 (0.2-3.2) Recess model OR=2.98 (1.1-8.1) Gross, 2007, Germany [48] cohort 1 year Hamon, 2003, France [49] cohort 2 years Prisco, 2000, Italy [50] cohort 1 year Ribichini, 2003, Italy [51] Guneri, 2005, Turkey [52] Okamura,1999 , Japan [53] Okumura, 2002, Japan [54] Ferrari, 2002, multicenter (Europe) [55] Koch, 2003, Germany [56] 81 (70/11), Eur Whites, 59/2 (1.4), CAD patients undergoing repeat PTCA for in-stent restenotic lesions at FU angiography 1010 (813/197), Eur Whites, 64 (11), symptomatic CAD patients treated with successful PTCA-STENT 29 (nr), Eur Whites, 60 (46-75), MI patients undergoing primary PTCA Recurrent Restenosis (1) [surrogate] Major adverse cardiac event (6) [clinical] Endothelial dysfunction (7) [surrogate] PTCA-STENT No 31 Allele contrast OR=1.03 (0.5-2.0) Domin model OR=0.51 (0.1-2.9) Recess model OR=1.38 (0.5-3.9) No association between ACE genotype and clinical outcome was observed ACE DD genotype was significantly (P=0.04) associated with an increase of PAI-1activity post PTCA [recess model OR= 6.52 (4.8-8.2)]. No significant gene-gene interaction was observed PTCA-STENT No 33 PTCA-STENT Yes (AGT1R, PAI-1) 34 No 35 Data synthesized in the metaanalysis Cohort 6.3 (2.5) months cohort 9 months (2.9) cohort 6 months 271 (nr), Eur Whites, 61 (10), CAD patients undergoing PTCA Restenosis (3) [surrogate] Angioplasty with ACEi treatment PTCA-STENT + ACEi 94 (59/35), Turks, 59.6 (9.9), CAD diabetic patients undergoing PTCA for stable angina pectoris 97 (84/13), East Asians, 60 (2), CAD patients undergoing PTCA for stable angina pectoris Restenosis (3) [surrogate] PTCA-STENT + ACEi No 26 Data synthesized in the metaanalysis Restenosis (3) [surrogate] No 29 Data synthesized in the metaanalysis cohort 6 months 92 (73/19), East Asians, 64.3 (8.9), CAD patients undergoing PTCA Restenosis (3) [surrogate] No 22 Data synthesized in the metaanalysis cohort 6 months 154 (119/35), Eur Whites, 61 (9.9), CAD patients undergoing PTCA Restenosis (1) [surrogate] PTCA-balloon + Imidapril 5 mg PTCA-STENT + Quinapril 18mg PTCA-STENT + ACEi No 36 Data synthesized in the metaanalysis cohort 1 year 612 (455/157), Eur Whites, 64.3 (9.5 ), CAD patients with DD genotype undergoing PTCA Restenosis (3) [surrogate] Restenosis (8) PTCA-STENT + ACEi No 36 D homozygotes receiving ACEi were not at a higher risk of angiographic or clinical restenosis [clinical] than patients who were not treated with ACEi (p=0.55) Meurice, 2001, France [57] cohort 6 months 79 (67/12), Eur Whites, 58.9 (10.6), CAD patients with DD genotype undergoing PTCA Restenosis (3) [surrogate] PTCA-STENT + Quinapril 20mg No 32 D homozygotes receiving Quinapril presented a trend towards increased restenosis compared to placebo Data synthesized in the metaanalysis Jorgensen, 2001, Netherlands [58] Toyofyuku, 2002, Japan [59] cohort 6 months 369 (293/76), Eur Whites, 59 (43-73), CAD patients undergoing PTCA for stable angina Restenosis (3) [surrogate] PTCA-STENT + ACEi No 40 RCT 6 months 204 (147/57), East Asians, 63.1 (1.0), CAD patients undergoing PTCA randomized to quinapril and placebo Restenosis (3) [surrogate] PTCA-STENT + Quinapril 20mg Yes (AGT) 30 No significant interaction between ACE polymorphism and quinapril treatment was observed on the risk of restenosis. No significant gene-gene interaction was observed Allele contrast OR=2.19 (0.9-4.8) Domin model OR=4.78 (0.638.3) Recess model OR=2.33 (0.8-6.7) Allele contrast OR=0.90 (0.4-1.9) Domin model OR=1.17 (0.2-5.7) Recess model OR=0.71 (0.2-2.4) Coronary Atherectomy Haberbosch, 1997, Germany [60] cohort 12-18 months 104 (91/13), Eur Whites, 60.2 (nr), CAD patients undergoing DCA and PTCA Restenosis (3) [surrogate] DCA-PTCASTENT No 26 Canosi, 2004, Italy [61] Cohort 6.3 (1.5) months 113 (103/10), Eur Whites, nr, CAD patients undergoing DCA and PTCA Restenosis (3) [surrogate] DCA-PTCASTENT No 29 CABG Dayi, 2005, Turkey [62] crosssectional 87 (71/16), Turks, 64.3 (7.3), CAD patients treated with CABG before >5 years undergoing angiography for symptoms Venous graft atherosclerosis (9) [surrogate] CABG No 28 Ortlepp, 2001, Germany [63] cohort 88 (52) months 101 (86/15), Eur Whites, 64.0 (8.1), CAD patients after CABG presenting for angiography due to angina pectoris Venous graft atherosclerosis (10) CABG No 33 Allele contrast OR=2.41 (1.3-4.5) Domin model OR=3.76 (1.212.2) Recess model OR=3.75 (1.311.3) The ACE I/D polymorphism was not associated with by-pass degeneration [surrogate] Volzke, 2002, Germany [64] cohort 2 years 247 (202/45), Eur Whites, 64.9 (nr), CAD patients undergoing CABG - total mortality - cardiac mortality or need for recurrent revascularization [clinical] CABG No 35 Voors, 2004, Netherlands [65] Cohort 12 months 82 (71/11), Eur Whites, 61.5 (1.3), CAD undergoing CABG endothelial dysfunction (11) [surrogate] CABG +quinapril (40mg) No 27 ACE I/D genotype is an independent predictor of midterm total mortality after CABG [Mortality rate: II=0%, ID=11.2%, DD=14.1%, p<0.05]. The ACE I/D genotype was also an independent predictor of the secondary end-point [cumulative cardiac event incidence: II=5.8%, ID=9.4%, DD=30.3%, p<0.005] quinapril completely restored the decreased vascular response in DD-genotype patients to the same level as II/ID genotype patients, while no effect of quinapril was demonstrated in the II/IDgenotype patients. Quinapril also prevented the increase in plasma ACE activity after CABG, especially in DD homozygotes Table 2. Invasive treatment study characteristics * Outcome definition criteria: 1. Restenosis defined as >50% progression of the residual stenosis at FU angiography compared with the findings immediately after PTCA, 2. Diagnostic criteria non-reported, 3. Restenosis defined as diameter stenosis >50% at FU angiography, 4. Endothelial dysfunction defined as coronary endothelium dependent vasomotion after intracoronary infusions of acetylcholine, 5. Clinical restenosis defined as death from cardiac causes, MI attributable to target vessel and target vessel revascularization, 6. Major adverse coronary event defined as death, MI, UA and coronary revascularisation, 7. Endothelial dysfunction defined as plasminogen activator inhibitor activity post-PTCA, 8. Clinical restenosis defined as need for target vessel revascularization due to symptoms or signs of ischemia in the presence of angiographic restenosis over one year after the intervention, 9. Venous graft atherosclerosis defined as total occlusion of venous graft, 10. Venous graft atherosclerosis defined by Gensini by-pass degeneration score, 11. Endothelial dysfunction defined as maximal vasoconstriction to angiotensin II. Abbreviations: DCA: directional coronary atherectomy, FU: follow-up, UA: unstable angina, RCT: randomized controlled trial.