PRINCIPLES OF PHARMACOLOGY
advertisement

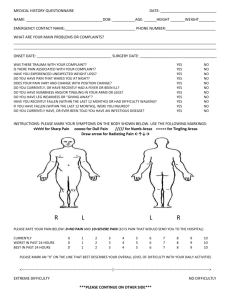
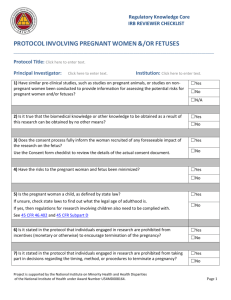
INTRODUCTION TO RESPIRATORY CARE PHARMACOLOGY I. Pharmacology and the Study of Drugs a. Drug i. A chemical which alters an organism’s function or processes 1. oxygen 2. alcohol 3. heparin 4. epinephrine 5. vitamins b. Pharmacology i. the study of drugs (chemicals), including their origin, properties, and interactions with living organisms c. Pharmacy i. The preparation and dispensing of drugs d. Pharmacognosy i. The identification of sources of drugs, from plants and animals e. Pharmacogenetics i. The study of the interrelationship of genetic differences and drug effects f. Therapeutics i. The art of treating disease with drugs g. Toxicology i. The study of toxic substances and their pharmacological actions, including antidotes and poison control II. Legislation Affecting Drugs a. See text, page 3, Box 1-1 b. Qualified medical practitioners who prescribe, dispense, or administer drugs must comply with federal and state laws governing the manufacture, sale, possession, administration, and dispensing and prescribing of drugs. c. All drugs available for legal use are controlled by the Federal Food, Drug and Cosmetic Act. This law protects the public by insuring the purity, strength, and composition of food, drugs, and cosmetics. It also prohibits the movement, in interstate commerce, of adulterated and misbranded food, drugs, devices, and cosmetics. d. Enforcement of this act is the responsibility of the Food and Drug Administration (FDA), which is part of the Department of Health and Human Services (HHS) of the US Government. e. The Controlled Substance Act of 1970 controls the manufacture, importation, compounding, selling, dealing in, and giving away of drugs that have the potential for addiction and abuse. These drugs are known as controlled substances (opium, cocaine, narcotics, stimulants, and depressants). The act is enforced by the Drug Enforcement Administration (DEA). Under federal law, medical practitioners who prescribe, administer, or dispense controlled substances must register with the DEA and physicians are required to renew their registration annually. f. Drug Schedules: controlled substances are classified according to five drug schedules i. Schedule I: have a high potential for abuse and are not accepted for medical use within the US ii. Schedule II: have a high potential for abuse (psychological or physical dependency) but do have an accepted medical use within the US iii. Schedule III: have a low to moderate potential for physical dependency with a high potential for psychological dependency iv. Schedule IV: have a low potential for abuse which may lead to limited physical or psychological dependency v. Schedule V: have the lowest abuse potential of the controlled substances consisting of antitussives and antidiarrheals vi. Examples of drugs Schedule I Heroin, LSD, marijuana, mescaline, peyote, and psilocybin Schedule II Amobarbital, amphetamine, cocaine, codeine, hydromorphone, opium, meperidine, methadone, morphine, Nembutal, pentobarbital, percodan, Quaalude, secobarbital, and Marinol Schedule III Barbituates, drug combinations including codeine and paregoric, gleutethimide, mazindol, methyprylon, phenmetrazine, and amphetamine like compounds Schedule IV Chloral hydrate, diazepam, meprobamate, paraldehyde, Phenobarbital, Librium, valium, darvon Schedule V Lomotil, donnagel, drugs containing low-strength codeine (Actifed with codeine) g. Federal law requires that all controlled substances be kept separate from other drugs under a double lock. The medicine nurse or charge nurse keeps the narcotic key during the shift. A separate record book is kept for narcotic counts which are done and shift change by an oncoming and off going nurse who both count the meds and sign for accuracy or discrepancy. III. Naming Drugs a. Chemical name i. The name indicating the drug's chemical structure b. Code name i. A name assigned by a manufacturer to an experimental chemical that shows potential as a drug 1. SCH 1000 – ipratropium bromide c. Generic name i. The name assigned by U.S. Adopted Name Council when the chemical appears to have therapeutic use and the manufacturer wishes to market the drug ii. The name is loosely based on the chemical structure iii. Drugs in the same class end in the same syllable iv. Written in lower case d. Official name i. The generic name becomes the official name when it is approved by the United States Pharmacopeia-National Formulary (USP-NF) e. Trade name i. The brand name given by a manufacturer ii. The name is capitalized and may have a trademark (®) IV. Sources of Drug Information a. United States Pharmacopeia-National Formulary (USP-NF) i. Gives standards for drugs in US ii. Contains fully approved drugs (by FDA) and formulas for drugs and mixtures b. AMA Drug evaluations i. Contains new drugs not officially in USP yet (in trials, animal and human) c. Physician’s Desk Reference (PDR) i. Prepared by manufacturers of drugs ii. Includes descriptive color charts for durg identification, names of manufacturers, and general drug actions iii. Published annually V. Sources of Drugs a. Animal i. thyroid hormone ii. insulin iii. pancreatic dornase b. Plant i. atropine ii. digitalis iii. curare c. Mineral i. magnesium sulfate ii. mineral oil d. Chemical synthesis i. manufactured in the laboratory VI. Process of Drug Approval in the United States a. Isolation and identification of a chemical with the potential for useful physiologic effects i. The exact structure and physical and chemical characteristics of an active ingredient are established b. Animal Studies i. general effects on the organism ii. effects on specific organs iii. toxicology studies for 1. mutagenicity – mutations 2. teratogenicity - potential to damage a fetus in utero 3. effect on fertility 4. carcinogenicity - causing cancer c. Investigational New Drug (IND) approval i. An application is filed with the FDA which contains 1. all information previously gathered 2. plans for human studies ii. Three years for human studies 1. Phase I - small group of healthy volunteers 2. Phase II - small group of subjects with the disease 3. Phase III - large multicenter studies to establish safety and efficacy d. New Drug Application (NDA) is filed with the FDA i. Upon approval, the drug is released for general clinical use e. This an involved, lengthy and expensive process i. The process can take an average of 13 - 15 years ii. The average cost is $350 million dollars iii. 1 in 10,000 identified chemicals will reach general clinical use f. Food and Drug Administration New Drug Classification System i. Chemical/Pharmaceutical Standing 1. New chemical entity 2. New salt form 3. New dosage form 4. New combination 5. Generic drug 6. New indication ii. Therapeutic Potential 1. A=Important therapeutic gain over other drugs 2. AA=Important therapeutic gain, indicated for a patient with acquired immunodeficiency syndrome (AIDS); fasttrack 3. B=Modest therapeutic gain 4. C=Important options; little or no therapeutic gain g. Orphan Drugs i. A drug or biological product for the diagnosis or treatment of a rare disease (< 200,000 persons) ii. A drug or biological product used with no reasonable expectation of recovering the cost of drug development VII. The Prescription a. The written order for a drug, along with any specific instructions fort compounding, dispensing, and taking the drug b. Parts of the prescription i. Patient’s name, address, and date ii. Superscription - Rx (meaning “recipe” or “take thou”) directs the pharmacist to take the drug listed and prepare the medication iii. Inscription – lists the name and quantity of the drug being prescribed iv. Subscription – the directions to the pharmacist on preparing the medication v. Transcription - Sig (signa) means “write”. The information the pharmacist writes on the label of the medications as instructions to the patient. vi. Name of the prescriber – the physician’s signature c. Over-the-Counter Drugs i. Drugs available without a prescription ii. The strength and amount per dose may be less than with a prescription formulation iii. OTC drugs can be hazardous in normal amounts if their effects are not understood and can be taken in large quantities d. Generic Substitution in Prescriptions i. A physician can indicate to the pharmacist that generic substitution is permitted in the filling of the prescription ii. The manufacturer of the generic substitute has not invested the considerable time and money required to develop an original drug product iii. The generic substitute will be less expensive to the consumer e. Common abbreviations used for prescriptions f. FDA Use-in-Pregnancy Ratings i. The FDA pregnancy categories are based on the degree to which available information has ruled out risk to the fetus, balanced against the drug’s potential benefits to the patient CATEGORY INTERPRETATION A Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities to the fetus in any trimester of pregnancy. B Animal studies have revealed no evidence of harm to the fetus, however, there are no adequate and well-controlled studies in pregnant women. OR Animal studies have shown an adverse effect, but adequate and wellcontrolled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester. C Animal studies have shown an adverse effect and there are no adequate and well-controlled studies in pregnant women. OR No animal studies have been conducted and there are no adequate and well-controlled studies in pregnant women. D Adequate well-controlled or observational studies in pregnant women have demonstrated a risk to the fetus. However, the benefits of therapy may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. X Adequate well-controlled or observational studies in animals or pregnant women have demonstrated positive evidence of fetal abnormalities or risks. The use of the product is contraindicated in women who are or may become pregnant