Supplementary Materials
advertisement

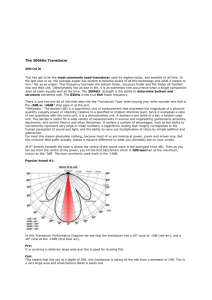
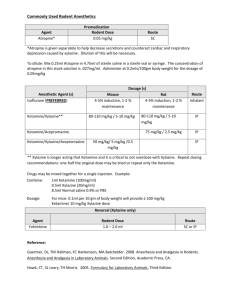
Controllable in vivo hyperthermia effect induced by pulsed high intensity focused ultrasound with low duty cycles Supplementary Materials I. Ultrasound Exposure System The 1.17-MHz HIFU transducer comprised an air-backed, 34.9-mm diameter APC880 PZT disk (APC International, Ltd., Mackeyville, PA, USA) mounted inside the transducer housing, with electrical connections on each surface of the PZT disk. The transducer housing consisted of an aluminum focusing lens with a 5-cm radius of curvature. The driving electronics consisted of a waveform generator (33120A, Agilent Technologies, Palo Alto, CA, USA) and an RF power amplifier (AP-400B, ENI, Rochester, NY, USA). Before the experiments, a membrane hydrophone (MHA 200A, NTR Systems, Inc., Seattle, WA, USA) was used to calibrate the spatial characteristics of the HIFU beam (-6 dB focal width of 4 mm). The maximum pressure amplitudes achievable were 27 MPa peak positive and 9 MPa peak negative. The ISPPA was determined by integrating the instantaneous intensity over 20 cycles: I SPPA 1 nT idt = 5300 W/cm2. nT During the experiments, the transducer was mounted to a water tank filled with degassed water. An acoustic absorber crafted from a rubber plate was positioned on the tank wall opposite the transducer to minimize the acoustic reflection. A custom-built polyamide cone filled with degassed water was screwed onto the transducer housing to provide acoustic coupling. The cone tip had a cutting-edge hole (1-cm diameter) that was covered with a polyurethane membrane (12.5-m thickness, Glad Products Co., Oakland, CA, USA) with an O-ring seal. The distance between the surface center of the focusing lens and the cone tip was 49 mm, permitting transducer’s focal region located at the cone tip and the exact focus of the HIFU beam sitting in the vessel. II. Animal Anesthesia Following initial sedation with a subcutaneous injection of an acetylpromazine (1.0 mg/kg)/ketamine (22 mg/kg) cocktail, the auricular surfaces were shaved and depilated to facilitate ultrasound coupling. A 21 gauge catheter was inserted into the proximal auricular vein of one ear through intravenous (IV) access. The animals were anesthetized with an IV ketamine (35-40 mg/kg)/xylazine (5 mg/kg) cocktail and placed in a lateral decubitus position in preparation for treatment. Before experiments, in vivo Doppler measurements were first preformed on rabbit auricular veins to determine the velocity of blood flow, whose average value was measured to be 4.25 cm/s. Rabbits remained anesthetized throughout the entire experimental protocol. At the end of the experiment rabbits were euthanized with an overdosed IV injection of ketamine/xylazine. The purpose of anesthetizing the rabbit was to successfully measure the steady-state temperature inside the rabbit auricular vein. All the animal preparation procedures were carried out according to the NIH guidelines and were approved by the University of Washington Animal Care and Use Committee. The injection dosage of the anesthesia drugs was determined by the body weight of the animal to insure safety and good anesthetic effect. It has been reported that an advantage of ketamine was the maintenance of spontaneous ventilation during anesthesiaS1. However, ketamine is rarely administered alone due to its poor muscle relaxation. It is most commonly combined with xylazine, acetylpromazine, or diazepam in a wide range of safety usages, including analgesia, anesthesia, hallucinations, and bronchodilationS2, for humans and small animals (e.g., cats, dogs, rabbits,rats). Although the administration of the anesthesia drugs is possible to affect the heart rate and arterial blood pressure of individual animals, the impact might not be statistically significant as long as the appropriate injection dosage is employedS3,S4. Especially, since the focus of the current studies is the temperature variations in the rabbit auricular veins exposed to HIFU pulses rather than investigations on the cardiovascular, pulmonary or neural issues and the blood flow in the anesthetized rabbit auricular vein was measured with Doppler instruments right before the experiments, the influence of anesthesia effect could be reasonably ignored. References: S1. T. Aye, B. Milne, Can. J. Anaesth. 49, 283 (2002). S2. T. E. Peck, S. A. Hill, M. Williams (2008). Pharmacology for anaesthesia and intensive care (3rd edition). (Cambridge university press, Cambridge, UK, 2008), p. 111. S3. A. L. Yershov, B. S. Jordan, J. M. Fudge, M. A. Dubick, Veter. Anaesth. Analg., 34, 157 (2007). S4. C. Baumgartner, M. Bollerhey, J. Ebner, L. Laacke-Singer, T. Schuster, W. Erhardt, Can. J. Vet. Res. 74, 200, (2010).