Dental radiology
advertisement

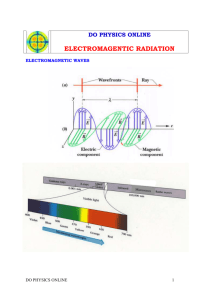
Lec. 1 Dental radiology Introduction Our wonderful universe is made of two main types of stuff. Matter which is made of atoms and molecules and radiation energy, which rapidly travels from one place to another till it, dissipates, which are interconvertible by Professor Albert Einstein. Matter: is any thing that occupies space and has mass. Radiation: is a form of energy transmits through space and matter. It may occur in two forms: stream of particles and electromagnetic waves. Radiology: is the medical science that deals with the study of radiation and its use, radioactive substances and other forms of radiation energy in the diagnosis and treatment of diseases. Radiograph: is an image on a film which is visible when viewed under transillumination, produced by the passage of X-ray through an object or body. Radiography: is the art and science of making radiographs by the exposure of films to Xrays. Dental radiology: is that area of dentistry which is concerned with the study of radiation and its use; for the evaluation of teeth, their associated structures, and facial and cranial bones. Dental radiograph: is an image on a film showing teeth and associated structures when viewed under transillumination. History of radiology 1831 1861 1895 1896 Mischael Faraday described electromagnetic induction. Leonard proposed inverse square law pertaining to the cathode rays. On November 8, Wilhelm Roentgen discovered X-ray. William T.G. Morton, a dentist, announced that it is possible to take radiograph of the teeth. 1896 Dr. Edmund C. Kells discovered his own X- ray apparatus and described a technique for radiographing the teeth and the jaw. He also proposed the principle of keeping the film and object at right angles to the source of the X-ray. 1896 Frank Harrison, an English dentist made dental radiographs with 10 minutes of exposure time. His images could differentiate the tooth structure and pulp chamber. He also reports the harmful effects of radiation. 1904 Weston A. Price a Cleveland dentist described paralleling technique and bisectingangle technique. 1918 Eastman Kodak Company developed a darkroom with processing tanks. 1925 Raper proposed bite-wing technique for the detection of interproximal caries. 1960 Panoramic X-ray machines were marked. 1986 Kinos L.T. ET all developed a portable radiographic X-ray camera made of tantalum and aluminum. Atomic structure The atom consists of a central nucleus and orbiting electrons. The nucleus consists of two primary subatomic particles protons positive electric and neutrons electrically neutral. The number of protons and neutrons in the nucleus of an atom determine its atomic weight. The numbers of protons is equal to number of orbiting electrons and determine its atomic number. Electrons are tiny negatively charged, the paths of electrons around the nucleus in orbits or shells, which lie at deferent distances from the nucleus. An atom has a maximum of seven shells (K, L, M, N, O, P, and Q). These shells represent different energy levels. I The electrons are held in place in the orbit by the electrostatic force between the positive nucleus and negative electrons. The binding energy is the distance between the nucleus and the orbiting electrons, so the electrons in the K shell have a greater binding energy than the electrons in the outer shell. This binding energy is measured in electron-volts (eV) or kiloelectron-volts (keV). The atoms can exist in a neutral or in an electrically unbalanced state. An atom is referred to as neutral atom when it contains equal number of protons and electrons. An atom with incompletely filled outer shell is electrically unbalanced and tries to gain electron to achieve a stable state. If an atom gains an electron it will have more negative charge, but when loss electron it will have more positive charge. An atom which has gained or lost an electron is called an ion. II Ionization: is the process of converting an atom to ion. An ion pair is formed when an electron is removed from an atom in the ionization process and become positive ion. This ion pair tries to attain electrically stable state by reacting with other ion. Radioactivity: is the process by which certain unstable atoms or elements undergo spontaneous disintegration or decay. A radioactive substance gives off energy in the form of particles or ray as a result of disintegration of atomic nuclei. Ionizing radiation Ionizing radiation is radiation that is capable of producing ions by removing or adding electron to an atom. Ionizing is grouped into: a) Particulate radiation b) Electromagnetic radiation. Particular radiation Particulate radiations are tiny particles of matter possessing mass and moving at high velocity in straight lines. There are three types of particles: Alpha particles are emitted from the nucleus of heavy metals and exist as two proton and neutrons, without any electrons. Accordingly, they quickly give up their energy and penetrate only a few microns of body tissue. Beta particles are fast-moving electrons emitted from the nucleus of radioactive atoms. Highspeed beta particles are able to penetrate matter to a greater depth than alpha particle, to a maximum of 1.5cm in tissue. This deeper penetration occurs because beta particles are smaller and lighter and carry a single negative charge; therefore they have a much lower probability of interacting with matter than do alpha particles. Beta particles are used in radiation therapy for treatment of skin lesions. Cathode rays are high-speed electrons originating in manufactured devices e.g. X-ray tube. Electromagnetic radiation It is the propagation of wave-like energy through space or matter. Electromagnetic radiations are either artificial or natural which are the cosmic rays, gamma rays, X-rays, ultraviolet rays, visible light, infrared light, radar waves, microwaves, and radiowaves. Electromagnetic spectrum is grouped of electromagnetic radiations according to there energies. The effect of electromagnetic radiation on human tissues as follow: Television and radiowaves: No effect on human tissues. Microwaves (low energy radiation): May produce heat within organic tissue. Low energy radiation capable of causing less ionization: As these have low ionization effects on living tissues, which used in magnetic resonance imaging. Gamma rays and X-rays: these rays are capable of causing ionization. X-radiation: is a high-energy ionizing radiation produced by the collision of a beam of electrons with a metal target in an X-ray tube as electromagnetic radiation. X-ray: is a beam of energy that has the power to penetrate substances and record the resultant image on radiographic film. X-rays have properties of both waves and particles. III Properties of X-rays 1. X-rays are invisible and cannot be detected by any of human senses. 2. X-rays have no mass or weight. 3. X-rays have no charge. 4. X-rays travel in the same speed as light. 5. X-rays travel as waves and have short wavelength. 6. X-rays have a high frequency. 7. X-rays travel in straight lines and can be deflected or scattered. 8. X-rays diverge from the source. 9. X-rays cannot be focused to a point. 10. X-rays have penetrating power. 11. X-rays are absorbed by matter. The absorption depends on the atomic structure of matter and the wavelength of the X-rays. 12. X-rays cause ionization of matter. 13. X-rays can cause certain substances to fluoresce or emit radiation in longer wavelength. 14. X-rays can produce image on photographic film. 15. X-rays can cause biologic changes in living cells. 16. An electrical and magnetic fields fluctuate perpendicular to the direction of X-rays and at right angles to each other. IV Medical uses of X-ray The two types of X-rays used in health sciences can be classified as: I. Diagnostic X-rays are used to make the shadow pictures of teeth, face, and jaws, which helps us to look at calcified structures and evaluate their insides. II. Therapeutic X-rays are usually very powerful radiation beams coming from radioactive nuclides, cyclotrons or linear accelerators, which help us to literally burn away the malignancies of the jaws. The branch of oncology which deals with the use of therapeutic radiation is called radiotherapy. V