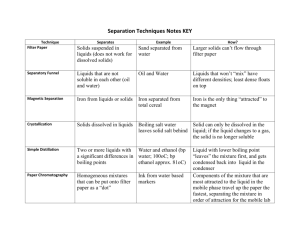
Mandatory Experiment 9.2a
advertisement

Mandatory Experiment 9.2a Determination of total suspended solids (expressed as p.p.m.) in a water sample by filtration Student Material Theory Insoluble substances that are too finely divided to settle may reduce light penetration in surface waters and interfere with plant and aquatic life. These substances may also affect fish life or they may indicate sewage discharges. The solids can consist of plant or animal matter and inorganic material such as sand. Determination of total suspended solids is done by firstly filtering a known volume of the water sample through small-pore filter paper. The increase in mass of the dry filter paper is then determined. Chemicals and Apparatus Water sample Small-pore filter paper Retort stand and clamp Filter funnel Large beakers (1000, 500, or 250cm3) Drying oven Balance Procedure NB: Wear your safety glasses. 1. Find the mass of a dry filter paper. 2. Filter 1 litre of the water sample. 1 3. Dry the filter paper at about 105 oC overnight. 4. Find the mass of the dried filter paper. 5. Calculate the mass of total suspended solids in mg/l (p.p.m.). Table of results Copy this table into your practical report book. Mass of dry filter paper before use Mass of the dried filter paper after filtration Mass of the suspended solids Volume of water sample Total suspended solids in mg/l (p.p.m.) = = = = = Questions relating to the experiment 1. Suggest some possible causes of high levels of total suspended solids. 2 2. What undesirable effects could result from high levels of total suspended solids? 3. How are these particles removed in water treatment? 3 Teacher Material A suitable water sample such as river or lake water where there is obviously a significant amount of solid matter in suspension should be used in this experiment. Tap water is unsuitable as it usually contains very low levels of suspended solids. If it is intended to use the filtered water for the total dissolved solids experiment, then a significant amount of salt could be added to the water sample before filtering. A suitable “recipe” is as follows: Add enough salt to 100 cm3 of hot water to make a saturated solution. Add this solution to the sample of dirty (cloudy) water. Small-pore filter paper such as glass-fibre (grade GF/C) is crucial to accuracy in this experiment. Larger-pore filter paper will allow smaller suspended solid particles to pass through. Vacuum filtration using a Buchner funnel and Buchner flask is recommended as it is not advisable to fold glass-fibre filter paper. If ordinary filter paper is to be used in this experiment, it should be dried in an oven prior to use. Only water samples with a high proportion of suspended solids should be used with ordinary filter paper. Whatman no. 2 and Whatman no. 4 filter paper have been found to give reasonably satisfactory results with samples of this kind. 4 Quantities per working group The volume of water sample will depend on the level of total suspended solids. 2 l for a water sample with low levels of total suspended solids and 1 l for a water sample with high level of total suspended solids should suffice. Safety considerations There are no major considerations except the obvious need to avoid toxic water samples. Disposal of wastes Discard used filter paper to refuse bin. Flush liquid residue to foul water drain. Sample Results and Calculations Mass of dry filter paper before use Mass of the dried filter paper after filtration Mass of the suspended solids Volume of water sample Total suspended solids in mg/l (p.p.m.) = 1.42 g = 1.60 g = 0.18 g =1l = 180 Suggested solutions to student questions 1. Suggest some possible causes of high levels of total suspended solids. Algal growth. Sandpit washings. Sewage discharges. 2. What undesirable effects could result from high levels of total suspended solids? Eutrophication. Damage to aquatic plants and animals. Sludge deposits. 3. How are these particles removed in water treatment? In settling tanks, the action of gravity allows much of the suspended solids to settle as sediment. The remainder are removed by filtration through sand supported by gravel. 5 Mandatory Experiment 9.2b Determination of total dissolved solids (expressed as p.p.m.) in a water sample by evaporation Student Material Theory Dissolved solids can affect the colour or taste of water and, if very high, may be an indication that the water sample is saline. Determination of total dissolved solids is done by evaporating a known volume of the filtered water sample. The increase in mass of the container is then determined. Chemicals and Apparatus Filtered water samples Drying oven 100 cm3 graduated cylinder 250 cm3 beaker Balance. Procedure NB: Wear your safety glasses. 1. Find the mass of a clean dry 250cm3 beaker. 2. Add 100cm3 of a filtered water sample to the beaker. 3. Evaporate to dryness in a dust-free oven at 105 oC. 4. Find the mass of the cool dry beaker. 5. Calculate the total dissolved solids in the water sample in mg/l (p.p.m.). 6 Table of results Copy this table into your practical report book. Mass of beaker Mass of beaker after the evaporation Mass of the dissolved solids Volume of water sample Total dissolved solids in mg/l (p.p.m.) = = = = = Questions relating to the experiment 1. Why must filtered water be used in this experiment? 2. Suggest some possible reasons for high levels of total dissolved solids. 3. A volume of 1200 cm3 of water was found to contain 0.09 g of dissolved solids. Express the concentration of the dissolved solids in p.p.m. 7 Teacher Material Sea water is particularly suitable for use in this experiment. Tap water is unsuitable as it usually contains very low masses of dissolved solids. The conventionally reported drying temperatures are 105 oC or 180 oC. At 105 oC the residue may contain some water even after one hour's drying. Some hydrogencarbonate will convert to carbonate by loss of CO2 but loss of organic matter will be minimal. At 180 oC virtually all the water plus some organic matter will be lost. Quantities needed per working group The amount of water sample required will depend on the water sample being used. 200 cm3 will generally be sufficient. Safety considerations There are no major considerations except the obvious need to avoid toxic water samples. Disposal of wastes Rinse with water and flush to foul water drain. Sample Results and Calculations Mass of beaker Mass of beaker after the evaporation Mass of the dissolved solids Volume of water sample Total dissolved solids in mg/l (p.p.m.) = 99.05 g = 100.54 g = 1.49 g = 100 cm3 = 14,900 Suggested solutions to student questions 1. Why must filtered water be used in this experiment? If the water has not been filtered, the result obtained will be the sum of the total suspended solids and total dissolved solids. 2. Suggest some possible reasons for high levels of total dissolved solids. The sample contains a high level of inorganic and /or organic soluble salts. This could indicate that the sample is saline or that the water basin contains naturally occurring minerals such as limestone. Alternatively a high level of total dissolved solids could be an indication of domestic, agricultural or industrial 8 pollution. 3. A volume of 1200 cm3 of water was found to contain 0.09 g of dissolved solids. Express the concentration of the dissolved solids in p.p.m. 1,200 cm3 ----------- 0.09 g 1,200 cm3 ----------- 90.0 mg 1,000 cm3 ----------- 75.0 mg 1,000 cm3 ----------- 75.0 p.p.m. 9 Mandatory Experiment 9.2c Determination of pH of a water sample Student Material Theory The most common range of pH present in fresh waters is 6.0 to 8.0. However, the range can extend from 4.5 for acidic, peaty waters to over 10.0 where there is intense, photosynthetic activity by algae. Water flowing over limestone tends to have its pH raised, due to the dissolving of calcium hydrogencarbonate in the water. Extremes of pH can affect the palatability and the corrosiveness of water as well as affecting plant and animal life. The pH of the water samples may be measured using a pH meter or pH paper or universal indicator solution. Chemicals and Apparatus Water samples pH meter or pH paper or universal indicator solution. Procedure NB: Wear your safety glasses. 1. Find the pH of each water sample by taking the respective readings on the pH meter, or by comparing the colours obtained using pH paper or universal indicator solution with the appropriate colour charts. 2. Record your results. Questions relating to the experiment 1. Suggest possible reasons for a relatively high pH value in a water sample. 2. Suggest possible reasons for a relatively low pH value in a water sample. 10 Teacher Material Quantities needed per working group Minimal. Safety considerations There are no major considerations except the obvious need to avoid toxic water samples. Disposal of wastes Flush to foul water drain. Suggested solutions to student questions 1. Suggest possible reasons for a relatively high pH value in a water sample. The water sample is very hard, due to running over limestone, or is subject to intense photosynthetic activity due to the presence of algae. 2. Suggest possible reasons for a relatively low pH value in a water sample. The water is acidic or peaty. Organic material decomposes to form acidic substances. 11