HOKLAS Requirement
advertisement

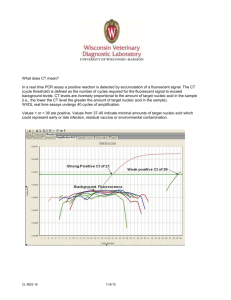
SC 30 Annex : Checklist on compliance with HOKLAS requirements – Molecular Pathology HOKLAS Requirement Clause SC 30 Annex – Page 1 of 3 Issue No. 3 * Y N NA QM Clause Remarks Technical requirements Accommodation and environmental conditions 5.2 For Nucleic Acid Amplification Testing ( NAT ) Are there separate rooms or clearly designated areas provided for the following 5.2.6 processes: ● A. sample reception; B. sample preparation and extraction; C. preparation of reagents and dispensing of master mix; and D. amplification and product detection? The distinct separation of these activities is to be maintained and appropriate procedures and control should be adopted to prevent cross-contamination. 5.2.6, 5.5.1 ● Is the nucleic acid samples kept in designated refrigerated compartments after sample 5.2.6, preparation but not kept at areas where activity such as gel electrophoresis or PCR 5.5.1 work is conducted? ● Is there a documented policy describing the movement of nucleic acid samples or specimens should as far as possible be unidirectional i.e. from pre-amplification to post-amplification areas? Laboratory equipment 5.3 For Nucleic Acid Amplification Testing ( NAT ) Note: 1. * The assessor should concentrate on items marked with a ●; other items will be checked by the team leader. 2. QM Clause stands for the clause number of the laboratory’s quality documentation that describes the laboratory’s procedures for the areas concerned. SC 30 Annex : Checklist on compliance with HOKLAS requirements – Molecular Pathology SC 30 Annex – Page 2 of 3 Issue No. 3 HOKLAS Requirement Clause * Are aerosol resistant pipette tips or positive displacement pipettes used in the whole process? 5.3.2 ● Is there a documented policy preventing the sharing of general instruments 5.3.8, (micropipettes, vortex mixers, heating block and micro-centrifuges) among designated 5.3.12 areas? Y N NA QM Clause Remarks ● A. sample reception; B. sample preparation and extraction; C. preparation of reagents and dispensing of master mix; and D. amplification and product detection. Pre-examination procedures 5.4 Is there an aliquoting protocol that prevent cross-contamination of different specimens 5.4.11 and contamination of original specimen? ● Examination procedures 5.5 Are the nucleic acids extraction and purification protocols published in literature, if not, is the in-house procedure validated and documented? 5.5.1, 5.5.2 ● Is there a written policy for the short and long term storage of DNA and RNA extracts to prevent degradation? 5.5.1, 5.5.3 ● Assuring the quality of examination procedures 5.6 Does the laboratory have a quality control program covering the phases of the analysis: specimen receipt, nucleic acid extraction, nucleic acid quantification, endonuclease digestion, electrophoresis, transfer, hybridization, detection, in situ hybridization, enzymatic amplification, photography and storage? 5.6.1 ● Note: 1. * The assessor should concentrate on items marked with a ●; other items will be checked by the team leader. 2. QM Clause stands for the clause number of the laboratory’s quality documentation that describes the laboratory’s procedures for the areas concerned. SC 30 Annex : Checklist on compliance with HOKLAS requirements – Molecular Pathology SC 30 Annex – Page 3 of 3 Issue No. 3 HOKLAS Requirement Clause * Has the laboratory made an effort to estimate the Uncertainty of Measurement for quantitative molecular tests such as viral load monitoring for HIV, HCV, HBV? 5.6.2 ● Does the laboratory participate in external quality assurance programme(s) covering each of the molecular tests for each target gene/microorganism? 5.6.4, 5.6.5 ● Does the laboratory develop and participate in an interlaboratory comparison programme when formal proficiency testing programme is not available? 5.6.5 ● Post-examination process 5.7 Are the results of examinations reviewed by authorized personnel with appropriate qualification, training and experience? 5.7.1 ● Are the primary samples, DNA extract, RNA extract and amplified DNA products retained for a minimum of 2 weeks after reporting? 5.7.2 ● Are gel images and hybridization blots kept as part of the technical records retained? SC30 6.2 ● Reporting of results 5.8 Is an indication of the reporting limits given in test reports when the presence of inhibitors is detected in Nucleic Acid Amplification testing? 5.8.3 (k), 5.8.5 ● Are all reports, including those requiring clinical interpretation, reviewed and signed by appropriate personnel with appropriate qualification, training and experience? SC30 7.2, 7.3, 7.4 ● If the laboratory is performing a referred test from a pathologist and that the test requires clinical input from a qualified pathologist, does the report contain this mandatory remark: “Test results shown on this report required clinical interpretation and comments by a qualified pathologist of respective discipline”? SC30 7.5 ● Y N NA QM Clause Remarks Note: 1. * The assessor should concentrate on items marked with a ●; other items will be checked by the team leader. 2. QM Clause stands for the clause number of the laboratory’s quality documentation that describes the laboratory’s procedures for the areas concerned.