Example Standard Operating Procedure
advertisement
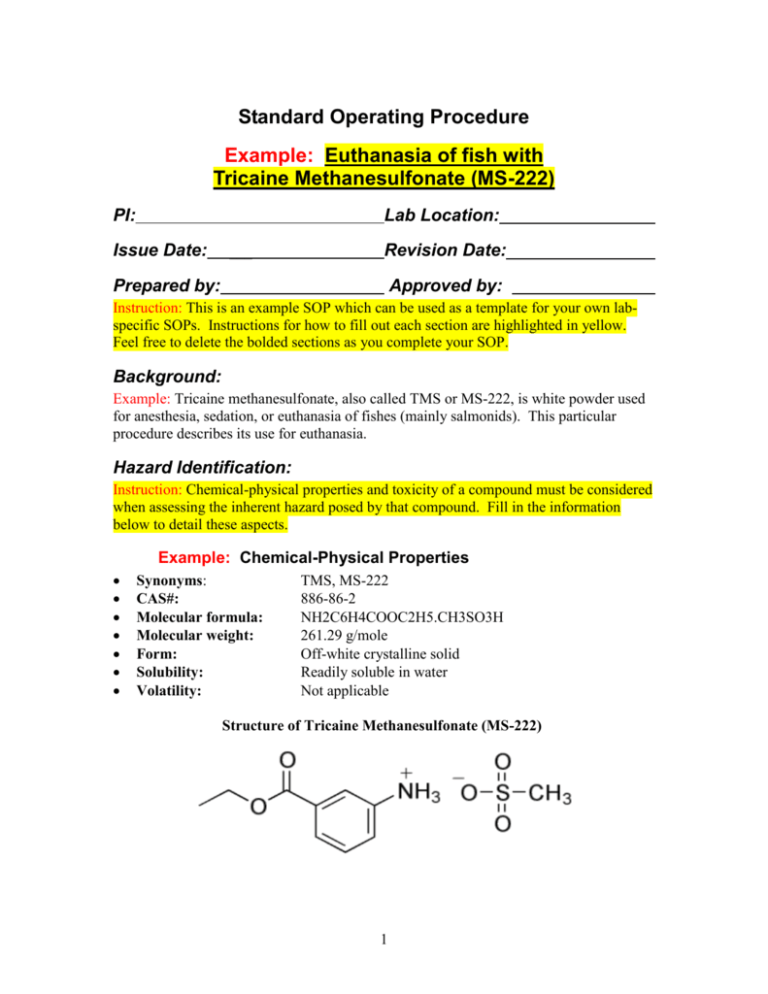
Standard Operating Procedure Example: Euthanasia of fish with Tricaine Methanesulfonate (MS-222) PI: Lab Location: Issue Date: ___ Revision Date: Prepared by: _ Approved by: ___________ Instruction: This is an example SOP which can be used as a template for your own labspecific SOPs. Instructions for how to fill out each section are highlighted in yellow. Feel free to delete the bolded sections as you complete your SOP. Background: Example: Tricaine methanesulfonate, also called TMS or MS-222, is white powder used for anesthesia, sedation, or euthanasia of fishes (mainly salmonids). This particular procedure describes its use for euthanasia. Hazard Identification: Instruction: Chemical-physical properties and toxicity of a compound must be considered when assessing the inherent hazard posed by that compound. Fill in the information below to detail these aspects. Example: Chemical-Physical Properties Synonyms: CAS#: Molecular formula: Molecular weight: Form: Solubility: Volatility: TMS, MS-222 886-86-2 NH2C6H4COOC2H5.CH3SO3H 261.29 g/mole Off-white crystalline solid Readily soluble in water Not applicable Structure of Tricaine Methanesulfonate (MS-222) 1 Example: Toxic Properties Acute effects: Chronic effects: Local effects: Systemic effects: Irritant to eyes, skin and respiratory tract. The lethal dose when delivered intravenously to mice was 180 mg/kg (LD50). TMS is a muscle relaxant that operates by preventing action potentials. Not available Very hazardous in case of skin contact (irritant, corrosive, sensitizer). Hazardous in case of eye contact (irritant) and inhalation (what effect?) and slightly hazardous in case of ingestion. Muscle relaxant – blocks action potentials and neurological signals between the extremities and the brain. Experimental Procedures: Instruction: Experimental procedures will affect potential exposure routes. Fill in details which will help determine the route and duration of exposure in this section (e.g. amount of chemical to be handled, how often, in what manner, etc.) Example: Tricaine Methanesulfonate (MS-222) is commonly used in the veterinary and research communities as an anesthetic for fish and amphibians. It is most commonly administered by adding it directly to the water in which the species is held and is already acclimated. For larger species, it can be effective by applying it directly to the gill area. For anesthesia, the optimum concentration used is 40-50 mg of TMS powder per liter of water. However, the optimum may vary with the size and species of the fish, and other variables. For euthanasia, MS222 is commonly used at a concentration of 250 mg/liter. The procedure is as follows: Based on the number and size of fish to be euthanized, determine the volume of water and amount of MS222 needed to prepare a solution of 250 mg/L tricaine methanesulfonate (TMS/MS222). Use water from the fish's tank in a volume generous enough to accommodate the fish. Wear personal protective equipment (PPE) – nitrile gloves, goggles and a lab coat – when handling any amount of MS222. Weigh the solid in a closed room to minimize air disturbances which might aerosolize the solid. Place wet paper towels around the balance to catch any dropped MS222 powder. Prepare the solution of 250 mg/L tricaine methanesulfonate (TMS/MS222) Check the pH of the solution and adjust if necessary (MS222 is acidic) with baking soda. Place the fish in the solution and leave until death is achieved (as determined by inactivity for 10 minutes.) Ensure death by decapitating the body. (See "Cornell University Cornell Center for Animal Resources and Education care306.01 Fish and Amphibian Euthanasia" www.research.cornell.edu/CARE/documents/SOPs/CARE306.pdf .) 2 The solution of TMS needs to be freshly prepared each time because TMS is lightsensitive and might form toxic by-products upon exposure to light. Researchers prepare this solution approximately once a month. Exposure Assessment: Instruction: Based on the activities you’ve described in the previous section, detail potential routes and duration of exposure in this section. Example: Route Inhalation: As the experimental procedure calls for solid material to be weighed on the bench top and respirators may or may not be worn, inhalation is a potential route of exposure. Experience with this procedure over several years has not resulted in exposure to date, so inhalation exposure is likely minimal. Eye absorption: Since the procedure mandates the researcher wear goggles, contact with eyes is unlikely. Skin Contact: The procedure mandates that the researcher wear a lab coat and gloves to prevent skin exposure. Therefore, contact with skin is unlikely. Ingestion: No eating or drinking is permitted in the laboratory. Therefore ingestion is unlikely. Injection: The procedure does not call for the use of sharps. Therefore accidental injection is unlikely. Example: Duration Frequency: Length: once a month <5 minutes to weigh out and dissolve the appropriate amount of solid. Risk Assessment: Instruction: Integrate the toxicity and exposures you’ve described in the previous sections to assess the degree of risk posed by the experimental procedure as you’ve written it. If you find the risks are unduly high, you may need to revise your experimental procedure and exposure assessment, and/or implement a control plan (in the following section) to reduce that risk. Detail your risk assessment in this section. Example: Due to the PPE specified in the experimental procedure, exposure through skin or eye contact is unlikely and associated risk is negligible. Risks are also negligible for ingestion and injection, as eating and drinking are prohibited in the laboratory, and needles are not used for work with MS222. There is some risk associated with inhalation, as researchers carrying out this procedure typically do not wear respiratory protection or work in the fume hood. However, the risk is likely to be low, as the amount of MS222 being handled is small (<250 mg), the duration of exposure is short (< 5 minutes/month), and at these levels the compound is not particularly toxic. 3 Control Plan: Instruction: Based on the degree of risk detailed in the previous section, describe control measures to reduce that risk in this section. Example: MS222 is also a skin and eye irritant and potentially toxic if inhaled at high doses. Therefore the following safety procedures should be followed: Under all conditions, wear nitrile gloves, goggles, and a lab coat when handling MS222. An N95 disposable respirator is not required for the application as described above, but is an inexpensive piece of protective equipment which will help prevent inhalation in the event of accidental aerosolization of solid MS222. Use is optional, and encouraged for those seeking additional protection, or who are new at carrying out the procedure. If weighing out larger quantities (>1 g) or weighing out more frequently (e.g. daily), wear an N95 disposable respirator to ensure workers are not accidently exposed to aerosolized MS222. Under these circumstances, researchers will need to participate in the university’s Respiratory Protection Program. Working in a fume hood would provide an even higher level of protection but air currents may make weighing the solid difficult. Waste Management Procedures: Example: Dispose of the MS222 solutions as hazardous waste unless you have obtained written permission to sewer it from DEHS (www.dehs.umn.edu/PDFs/disposal.pdf). Without written permission, collect waste mixtures in compatible container and label as Hazardous Waste. Spill and Accident Procedures: Instruction: Spills, by definition, are uncontrolled situations and therefore are likely to pose a much higher degree of risk than routine experimental procedures. Assess potential worst-case situations for your compound and detail procedures to follow in such emergencies in the following section. Example: Spill Cleanup Solid: If source container drops and breaks (1-100 grams max), avoid raising dust – do not sweep up dry material. Evacuate spill area, restrict others from entering the area and from a safe spot, call DEHS (612-626-6002) or 911 (after hours). If a small amount (<250 mg) spills outside of spill containment (the wet paper towels around balance), ensure personal protective equipment (PPE) is appropriate (nitrile gloves, goggles, and a lab coat), cover material with paper towels, spray lightly with water and carefully scoop material and paper towels into container for appropriate disposal (see Waste Management Procedures - above) Solution: If source container or treated fish tank breaks and spills, call DEHS. Prevent liquid from entering sewer. Apply absorbent pads for larger amounts of 4 liquid and, using appropriate PPE, carefully scoop wetted absorbent material into container for appropriate disposal (see Waste Management Procedures). Example: Accidental Exposures Inhalation: Allow the victim to rest in a well ventilated area. Seek immediate medical attention. Eye absorption: Check for and remove any contact lenses. Rinse eyes for 15 minutes in the eyewash. Seek medical attention. Skin Contact: If the chemical contacts the clothed portion of the body, remove the contaminated clothes as quickly as possible. Bag clothing for appropriate disposal (see Waste Management Procedures - above). If sewering if permitted, employer may wash and return clothing to worker. If the chemical contacts exposed skin, gently and thoroughly wash with running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and mucous membranes. If irritation persists, seek medical attention. Accidental ingestion: Do not induce vomiting. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth resuscitation. Seek immediate medical attention. Accidental injection: Not applicable References: http://www.sciencelab.com/msds.php?msdsId=9925304 http://en.wikipedia.org/wiki/Tricaine http://ccinfoweb2.ccohs.ca/rtecs/Action.lasso?-database=rtecs&-layout=Display&response=detail.html&-op=eq&RTECS+NUMBER=DG2455000&-search 5









![This article was downloaded by: [Oregon State University]](http://s2.studylib.net/store/data/010881326_1-423cbd75b0b228bbc400ac8651cb3851-300x300.png)